Table of Contents
Introduction
Semaglutide is a medicine that has quickly become popular for helping people manage type 2 diabetes and lose weight. It belongs to a group of drugs called GLP-1 receptor agonists. These drugs work by helping the body make more insulin, slow down digestion, and reduce hunger. Because of how well semaglutide works, many people have started using it. It is sold under brand names like Ozempic, Wegovy, and Rybelsus. While many people benefit from semaglutide, some concerns have come up about possible side effects, including effects on the eyes.
One rare but serious eye problem being discussed is retinal detachment. The retina is a thin layer of tissue in the back of the eye. It sends signals to the brain so that a person can see. If the retina pulls away from the back of the eye, it is called retinal detachment. This condition can cause sudden vision loss and must be treated quickly. Without treatment, it can lead to permanent blindness in the affected eye.
Recently, there have been questions about whether semaglutide could be linked to retinal detachment. Some reports from patients and doctors have suggested a possible connection. These reports do not prove that semaglutide causes the problem, but they raise enough concern to look deeper into the matter. When many people begin using a new medicine, researchers watch carefully to see if any unexpected health problems start to appear. This process is called post-marketing surveillance. It helps medical experts find rare side effects that might not have shown up in clinical trials.
Retinal problems are not new in people with diabetes. In fact, people with diabetes have a higher risk of eye diseases like diabetic retinopathy, which can lead to vision loss. Some eye problems happen more often when blood sugar levels change too quickly. Since semaglutide can lower blood sugar and lead to fast weight loss, researchers wonder if this sudden change could somehow affect the eye, especially the retina.
Most of the early concerns have come from case reports. These are detailed notes written by doctors when they see unusual medical events in patients. Case reports are important because they can be the first signs of a rare side effect. But they do not prove cause and effect. That is why more studies are needed to find out if semaglutide truly raises the risk of retinal detachment or if these events are happening by chance in people who already had other risk factors.
The purpose of this article is to explore what is known about the possible link between semaglutide and retinal detachment. It will look at how semaglutide works, what retinal detachment is, and whether there is any real connection between the two. It will also explain what science says so far, what questions still need to be answered, and what health professionals and patients should keep in mind.
As interest in semaglutide grows, so does the need to understand its full safety profile. While most people use the medicine without serious problems, even rare side effects must be taken seriously—especially if they can affect something as important as eyesight. The goal is not to cause fear but to provide clear, easy-to-understand information based on current research. Knowing the facts can help people make informed decisions about their health and give doctors the tools to monitor patients more carefully.
Understanding possible risks, even if small, can help protect vision and support better care. Whether or not a strong link between semaglutide and retinal detachment exists, learning about the topic is an important step for patients, doctors, and researchers alike.
What is Semaglutide?
Semaglutide is a medicine used to help control blood sugar levels and support weight loss. It is part of a group of drugs called GLP-1 receptor agonists. These drugs copy the action of a natural hormone in the body called glucagon-like peptide-1 (GLP-1). GLP-1 helps control how the body handles food, insulin, and blood sugar. Semaglutide was first approved to treat type 2 diabetes, but now it is also used to help people lose weight, even if they do not have diabetes.
How Semaglutide Works
Semaglutide works in several ways to help the body. First, it increases insulin when blood sugar is high. Insulin is a hormone that helps move sugar from the blood into the cells, where it can be used for energy. Semaglutide also lowers the amount of glucagon, another hormone that tells the liver to release sugar into the blood. When glucagon is lower, the liver releases less sugar.
Another important action of semaglutide is that it slows down the emptying of the stomach. This means food stays in the stomach longer, which helps people feel full for a longer time. This can help with appetite control and lead to weight loss. Semaglutide also acts on the brain to reduce hunger and food cravings.
These effects together make semaglutide helpful for both blood sugar control and weight management.
What Type of Medicine Is It?
Semaglutide belongs to a group of drugs called GLP-1 receptor agonists. These drugs copy the action of the GLP-1 hormone that the body makes naturally in the gut after eating. GLP-1 helps lower blood sugar and reduce appetite. Other drugs in the same group include liraglutide, dulaglutide, and exenatide. Semaglutide is one of the newer and more powerful drugs in this class. It works longer in the body than older GLP-1 drugs, so it only needs to be taken once a week in most cases.
Who Is It For?
Semaglutide is used for two main reasons:
- Type 2 Diabetes: Doctors prescribe semaglutide to help people with type 2 diabetes lower their blood sugar. It can be used alone or with other diabetes medicines. People with type 1 diabetes should not use semaglutide because it is not approved for that type of diabetes.
- Weight Management: Some versions of semaglutide are approved for use in people who are overweight or have obesity. It can help people lose a significant amount of weight when combined with healthy eating and physical activity.
The drug is not used to treat type 1 diabetes or diabetic ketoacidosis, a serious condition linked to high blood sugar. It is also not recommended for people with certain types of thyroid tumors or a family history of medullary thyroid cancer.
Brand Names of Semaglutide
Semaglutide is sold under different brand names, depending on how it is used:
- Ozempic: This version is used to treat type 2 diabetes. It is a weekly injection.
- Wegovy: This version is used for weight loss. It also comes as a weekly injection but in different doses than Ozempic.
- Rybelsus: This version is a daily pill taken by mouth. It is used for type 2 diabetes.
Even though all three contain semaglutide, they are approved for different uses and come in different forms and doses. It is important for patients and healthcare providers to choose the right one based on the treatment goal.
Benefits and Common Side Effects
Semaglutide offers many benefits. For people with diabetes, it helps lower blood sugar and may reduce the risk of heart problems. For people trying to lose weight, it can help reduce body weight significantly over time. Many people also experience lower blood pressure and improved cholesterol levels.
However, semaglutide can cause side effects. The most common ones include:
- Nausea
- Vomiting
- Diarrhea
- Constipation
- Stomach pain
These side effects are usually mild and may go away as the body gets used to the medicine. Some people may also lose too much appetite or feel tired. Rare but serious side effects include:
- Inflammation of the pancreas (pancreatitis)
- Gallbladder problems
- Kidney problems due to dehydration
- Possible thyroid tumors (seen in animal studies)
Patients should be monitored by a healthcare provider, especially when starting treatment. Dose increases are usually done slowly to reduce stomach side effects.
Semaglutide is a powerful drug used to treat type 2 diabetes and obesity. It works by copying the body’s own hormones to lower blood sugar and reduce appetite. While it has many benefits, it also comes with possible side effects. Understanding how semaglutide works and who it is for helps explain why it is becoming more common in medical care today.
Understanding Retinal Detachment
The retina is a thin layer of tissue at the back of the eye. It plays a very important role in vision. Light enters the eye and passes through the lens. It then hits the retina, which changes the light into signals that are sent to the brain. The brain turns these signals into images, allowing a person to see.
What Is Retinal Detachment?
Retinal detachment happens when the retina pulls away from the back wall of the eye. When this occurs, the retina no longer gets the oxygen and nutrients it needs. If not treated quickly, retinal detachment can cause permanent vision loss in the affected eye.
There are three main types of retinal detachment:
- Rhegmatogenous Retinal Detachment
This is the most common type. It starts with a tear or hole in the retina. Fluid from the center of the eye (called the vitreous) leaks through this hole. The fluid collects under the retina, causing it to lift off or “detach” from the tissue underneath. - Tractional Retinal Detachment
This type is more common in people with diabetes. Scar tissue forms on the surface of the retina. Over time, the scar tissue shrinks and pulls on the retina. This can cause the retina to detach. - Exudative Retinal Detachment
This type does not involve a tear or scar tissue. Instead, fluid builds up beneath the retina because of inflammation, injury, or diseases like cancer. The fluid pushes the retina away from the back of the eye.
Each type of retinal detachment is serious and needs fast medical care.
Risk Factors for Retinal Detachment
Some people have a higher chance of developing retinal detachment. The main risk factors include:
- High Myopia (Severe Nearsightedness)
People who are very nearsighted have longer eyeballs. This makes the retina thinner and more fragile, especially at the edges. Thinner retinas are more likely to tear or develop holes. - Eye Injury or Trauma
A strong blow to the head or eye—such as from a sports accident or car crash—can cause the retina to tear or detach. - Previous Eye Surgery
Surgery to treat cataracts or other eye problems can raise the risk, especially if complications occurred during the procedure. - Family History
Having close family members who have had retinal detachment may increase a person’s risk. - Aging
As people get older, the gel-like fluid in the eye (called vitreous) shrinks. This can pull on the retina and lead to tears or detachment. Most retinal detachments happen in people over age 50. - Diabetic Eye Disease (Diabetic Retinopathy)
Long-term diabetes can damage blood vessels in the retina. Scar tissue can grow and pull the retina away from the back of the eye.
Symptoms of Retinal Detachment
Retinal detachment is a medical emergency. Symptoms can appear suddenly. They often include:
- Flashes of Light
Bright flashes in the side vision may feel like camera flashes or lightning bolts. These occur when the retina is being pulled. - New Floaters
Small dark shapes or spots that float across the vision may suddenly appear. These can look like dots, strands, cobwebs, or bugs. - A Shadow or Curtain Across Vision
A dark area or shadow may spread across part of the visual field. It may look like a curtain being pulled over the eye from the top, bottom, or side. - Blurred Vision or Sudden Vision Loss
As the retina pulls away, vision may become blurry. If too much of the retina detaches, central or full vision may be lost in that eye.
It is important to note that retinal detachment does not cause pain. That makes it harder for some people to know that something is wrong. Ignoring symptoms or waiting too long can lead to permanent vision loss.
Importance of Fast Treatment
Retinal detachment can often be treated if caught early. Eye doctors use procedures like laser therapy, freezing (cryopexy), or surgery to repair the retina. The goal is to seal the tear and reattach the retina to the back of the eye.
The chances of saving vision are much better when treatment happens within a few days. The longer the retina stays detached, the greater the risk of losing vision permanently.
Knowing the symptoms and acting quickly are key to protecting eyesight. People with risk factors—such as diabetes or high myopia—should have regular eye exams and report any vision changes right away.
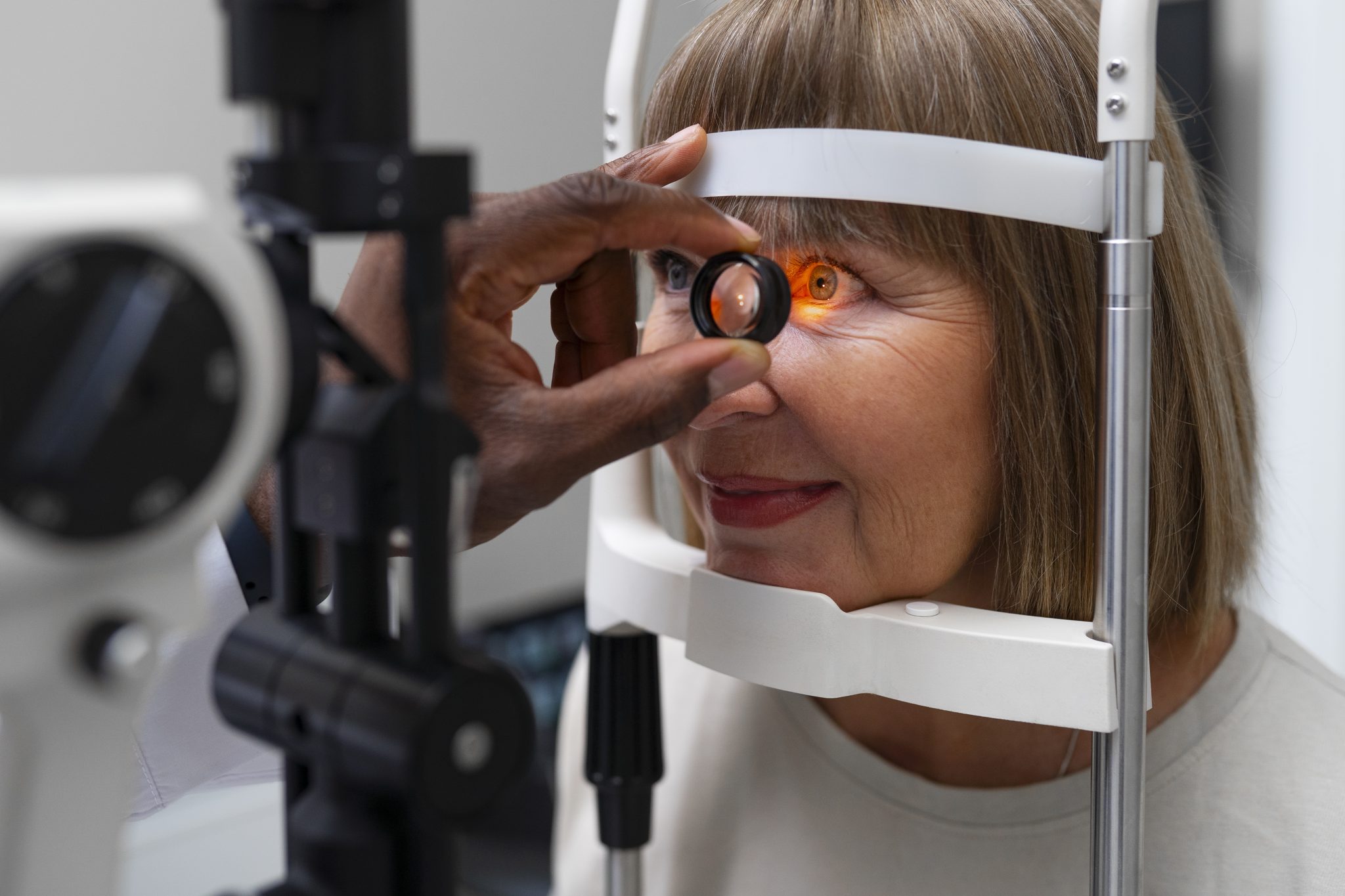
What is the Reported Link Between Semaglutide and Retinal Detachment?
Semaglutide is a medicine that helps lower blood sugar and support weight loss. It is often used by people with type 2 diabetes or obesity. While semaglutide has been shown to be helpful for many people, some health concerns have been raised, including possible effects on the eyes. One of the concerns that has come up is a possible link between semaglutide and a serious eye problem called retinal detachment.
Retinal detachment happens when the retina, which is the thin layer of tissue at the back of the eye, pulls away from the layer underneath it. This can lead to blurry vision, flashes of light, or even blindness if not treated quickly. Because the retina plays an important role in how people see, any condition that could affect it needs to be taken seriously.
Some doctors and researchers have started to notice a small number of patients who developed retinal detachment after starting semaglutide. These cases were not seen during the early studies of semaglutide, but they have been reported in real-world use after the medicine became more widely available. These reports have caused both health experts and patients to ask questions about whether semaglutide might somehow raise the risk of retinal detachment.
When a new medicine is released to the public, it continues to be watched closely. Health agencies collect reports of side effects through systems called pharmacovigilance databases. These systems include the U.S. Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) and similar systems in other countries. Doctors, pharmacists, and sometimes patients themselves can report any unusual health problems that happen while taking a medication.
In the case of semaglutide, a small number of these reports have mentioned retinal detachment. However, just because something is reported after using a medicine does not mean the medicine caused it. Many people who take semaglutide already have health problems like diabetes, which can damage the eyes over time. Some people may also have other risk factors such as high levels of nearsightedness (called myopia) or a history of eye surgery or trauma. These factors can make it hard to know whether semaglutide is truly linked to retinal detachment or whether it is just a coincidence.
One of the main problems with these reports is that they do not show a clear cause-and-effect relationship. For example, it is not always clear if the person was already having early signs of retinal damage before they started semaglutide. In other cases, the person may have had a retinal detachment weeks or months after taking the drug, which makes it difficult to know if there is any connection. Because these reports are often missing important medical details, experts cannot make firm conclusions just from the data alone.
So far, no large scientific studies have confirmed that semaglutide directly causes retinal detachment. In fact, in the clinical trials that tested semaglutide before it was approved, retinal detachment was not reported as a common side effect. However, those trials may not have included enough people or followed them long enough to detect rare events like retinal detachment.
Still, because any eye-related problem can be serious, researchers are paying close attention. Medical researchers are reviewing the data and looking for patterns, such as whether the cases of retinal detachment are happening more often in people taking semaglutide than in those who are not. They are also looking to see if these events are happening in people who have certain eye conditions to begin with.
At this point, there is no proof that semaglutide causes retinal detachment. However, the reported cases have raised important questions. Until more is known, doctors are being encouraged to ask their patients about any changes in vision and to report any eye problems to health agencies. Patients should also be aware of symptoms like seeing floaters, flashes of light, or a dark curtain in their vision, and they should seek care right away if these occur.
The possible link between semaglutide and retinal detachment is still being studied. More research is needed to understand whether the drug plays a role or whether the cases are due to other factors. For now, the reports highlight the need for careful monitoring and open communication between patients and their healthcare providers.
What Does the Scientific Literature Say?
Semaglutide is a medicine that helps lower blood sugar and body weight. It is often used in people with type 2 diabetes or obesity. As semaglutide becomes more popular, doctors and researchers are paying closer attention to possible side effects, especially those affecting the eyes. Some people have reported changes in their vision while using this drug, and a few have raised concerns about retinal detachment.
To understand if semaglutide can cause retinal detachment, scientists have looked at different types of studies. These include clinical trials, case reports, and data collected from real-world use.
Clinical Trials
Before semaglutide was approved for use, it was studied in several large clinical trials. These trials tested the drug in thousands of people to see if it was safe and effective. The most well-known trials include the SUSTAIN, STEP, and PIONEER studies.
The SUSTAIN trials focused on people with type 2 diabetes using semaglutide as an injection. The PIONEER trials studied the oral version of semaglutide. The STEP trials looked at people using the drug for weight loss, even if they did not have diabetes.
In these trials, many common side effects were found. These included nausea, vomiting, and stomach problems. Some people also reported vision changes. However, retinal detachment was not listed as a common or expected side effect in these trials. It is possible that the number of cases was too small to show a clear pattern. Also, people with serious eye problems were sometimes not included in these studies, which can make it harder to know the full risk.
Reports from the Real World
After a drug is approved and used by more people, new side effects can sometimes appear. Doctors and patients report unusual symptoms to safety systems like the U.S. Food and Drug Administration’s (FDA) MedWatch or Europe’s EudraVigilance database.
Some of these real-world reports have mentioned eye problems while using semaglutide. These include blurry vision, worsened diabetic retinopathy, and rare cases of retinal detachment. However, these reports do not prove that semaglutide caused the problem. They only show that the event happened after the person started using the drug.
A few case studies in medical journals have also described people who developed retinal problems after starting semaglutide. In these reports, symptoms such as flashes of light or sudden vision loss were noticed. In some cases, patients were later diagnosed with retinal detachment. But it is hard to tell if the drug was the cause or if other factors were involved, such as diabetes, age, or past eye surgeries.
Large Observational Studies
Researchers have also tried to study large groups of people using semaglutide by looking at their medical records. These studies are called observational studies. They help scientists find patterns that may not appear in small trials.
So far, only a few of these studies have looked closely at eye health. One study did find a possible increase in eye problems, especially in people whose blood sugar levels dropped quickly. This may be due to sudden changes in the eye’s blood vessels. However, this study did not confirm a direct link between semaglutide and retinal detachment.
Limits of Current Research
Many of the studies on semaglutide and vision do not have enough data about retinal detachment. Most trials were not designed to look for rare eye problems. Also, some people already had diabetic eye disease before starting the drug, which makes it harder to know what caused the problem.
Another challenge is that retinal detachment is rare. Even if semaglutide did increase the risk slightly, it would take many more studies to detect the change. There is also a lack of long-term data, especially for people using semaglutide for weight loss rather than diabetes.
At this time, there is no strong proof that semaglutide directly causes retinal detachment. Clinical trials have not shown a clear link. A few reports and small studies have raised questions, but more research is needed. The current evidence is not enough to confirm or deny a connection. Doctors and researchers are watching carefully to see if more cases appear over time.
Continued monitoring and new studies will help answer these questions more clearly in the future. For now, it is important to be aware of possible vision changes and to speak with an eye specialist if symptoms appear.
How Might Semaglutide Potentially Affect the Eye?
Semaglutide is a medication that helps lower blood sugar and support weight loss. It works by mimicking a hormone in the body called GLP-1 (glucagon-like peptide-1). This hormone affects how the body releases insulin and slows down digestion. While semaglutide is effective for managing type 2 diabetes and obesity, some people have raised concerns about how it may affect the eyes, including the possibility of retinal detachment.
Retinal detachment is a serious eye condition where the retina—the light-sensitive layer at the back of the eye—pulls away from its normal position. This can lead to vision loss if not treated quickly. There is currently no strong evidence that semaglutide causes retinal detachment directly, but some researchers are exploring how the drug might play a role in certain eye problems.
GLP-1 Receptors in the Eye
Researchers have found GLP-1 receptors in different parts of the eye, including the retina. These receptors are proteins that respond to the GLP-1 hormone or drugs that mimic it, like semaglutide. The exact role of GLP-1 receptors in the eye is still being studied, but it’s possible that semaglutide could affect the eye by acting on these receptors.
Some animal studies have shown that GLP-1 receptor agonists might reduce inflammation and protect nerve cells in the retina. This could be helpful, especially in people with diabetic eye disease. However, it’s not yet clear if these protective effects also happen in humans or if GLP-1 drugs might have other unintended effects on the eye, such as disturbing the retina’s normal structure or function.
Rapid Blood Sugar Control and the Retina
One theory is that rapid improvement in blood sugar levels can sometimes affect the eye, especially in people who already have diabetic eye disease. When someone starts a new diabetes treatment like semaglutide, their blood sugar may drop quickly. While this is good for overall health, sudden changes in blood sugar can sometimes cause temporary changes in the shape of the eye lens or in the small blood vessels of the retina.
These changes can lead to shifts in vision and may affect how the retina is attached. In rare cases, this could possibly lead to retinal complications. This kind of effect has been seen with other diabetes medications too, not just semaglutide. It is not considered a direct cause of retinal detachment, but it may create stress on the retina, especially if the person already has eye problems.
Vitreous Changes and Retinal Detachment
The vitreous is a clear gel that fills the inside of the eye and helps maintain its shape. Over time, especially with age or in people with diabetes, this gel can shrink or pull away from the retina. This is called posterior vitreous detachment (PVD). Sometimes, this pulling can cause the retina to tear or detach.
It is unclear whether semaglutide has a direct effect on the vitreous. However, if semaglutide leads to rapid weight loss or fast shifts in blood sugar, it may affect how the vitreous gel interacts with the retina. These changes could possibly increase the risk of a tear or detachment in people who are already at risk. This might include people who are very nearsighted, have had eye surgery, or have diabetic retinopathy.
What Is Known and What Is Not
At this time, there is no confirmed biological pathway that shows semaglutide directly causes retinal detachment. Most studies and reports do not show a strong link. However, the body is complex, and there may be rare or indirect effects that are not yet fully understood. More research is needed to study the eye in people taking semaglutide over time, especially those with other eye conditions.
Scientists are still working to understand how GLP-1 drugs affect different parts of the body. Since the eye is a sensitive organ with many small structures, even small changes in blood flow, fluid balance, or tissue pressure could have an impact.
While there is no clear proof that semaglutide causes retinal detachment, some changes in the eye related to blood sugar control or the vitreous could possibly increase risk in certain people. Anyone with a history of eye problems or diabetes-related eye disease may need to be monitored closely when starting semaglutide. More studies are needed to fully understand the long-term effects of this medication on the eye.
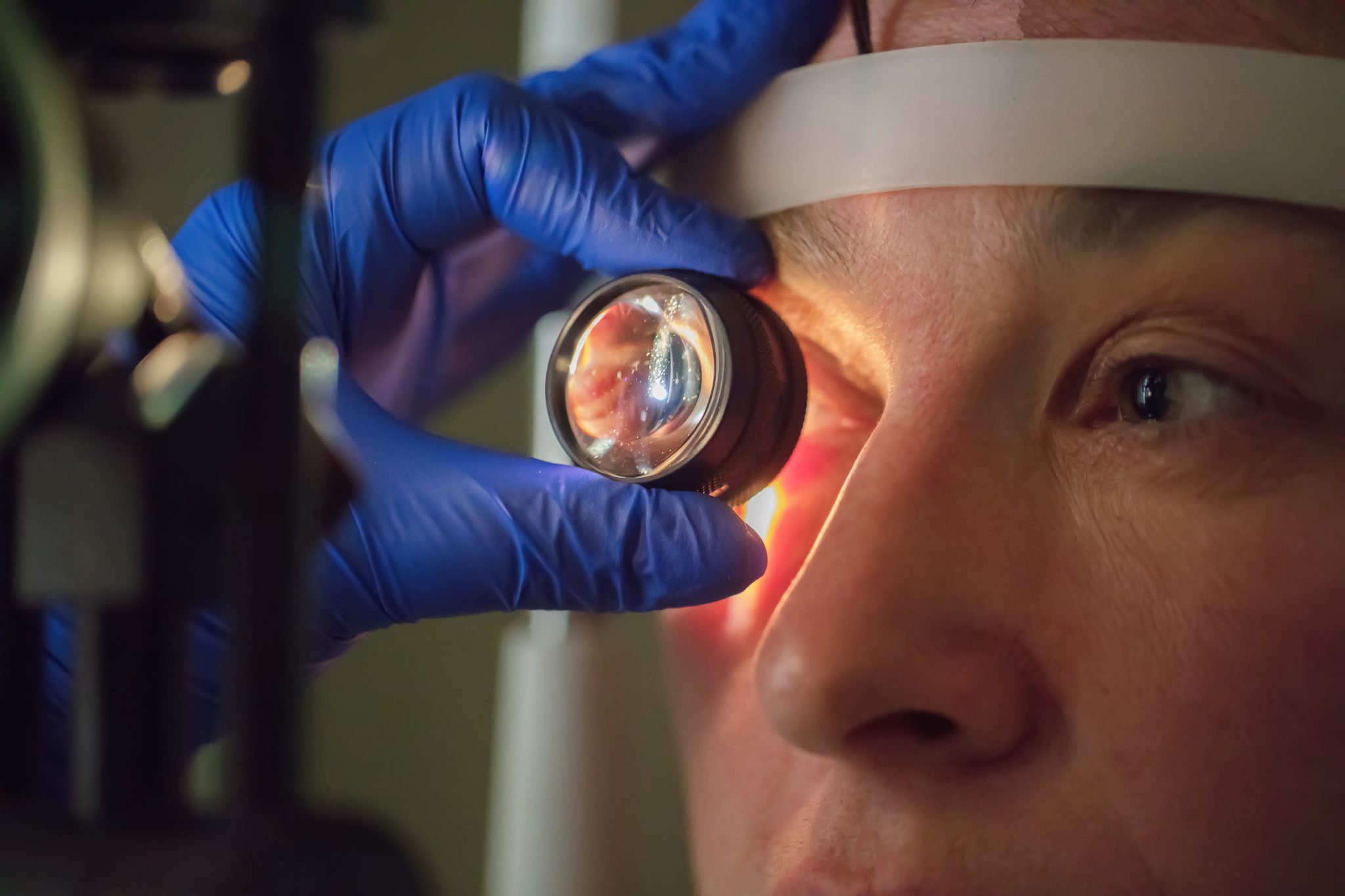
Are People With Diabetes or Myopia at Higher Risk?
Retinal detachment is a serious eye condition that can lead to vision loss if not treated quickly. Some people are more likely to develop this problem than others. Two groups often mentioned are those with diabetes and those with myopia (nearsightedness). These groups may also be more likely to take medications like semaglutide. Understanding how diabetes and myopia affect the eyes can help explain whether semaglutide could increase the risk of retinal detachment in these individuals.
Diabetes and Eye Health
Diabetes is a condition that causes high blood sugar levels over a long period. High blood sugar can damage many parts of the body, including the eyes. One of the most common eye problems caused by diabetes is diabetic retinopathy. This condition affects the small blood vessels in the retina, the thin layer of tissue at the back of the eye that helps with vision.
Over time, these blood vessels can swell, leak fluid, or close off completely. New abnormal blood vessels may also grow, which can bleed or scar the retina. These changes can lead to retinal detachment, especially when scar tissue pulls on the retina. People with diabetic retinopathy are more likely to have this type of detachment, called tractional retinal detachment.
The Role of Semaglutide in Diabetes Management
Semaglutide is used to lower blood sugar levels and help with weight loss in people with type 2 diabetes. It works by helping the body make more insulin and slowing down digestion. When blood sugar drops quickly, especially in someone with existing diabetic eye disease, it may cause sudden changes in the eye. Rapid improvements in blood sugar can sometimes make diabetic retinopathy worse before it gets better. This has been observed with other treatments that lower blood sugar quickly, such as insulin.
Although semaglutide helps control blood sugar, there is still ongoing research into whether it could affect the eyes in unexpected ways, especially in people with pre-existing diabetic eye disease. If a person already has changes in the retina, such as swelling or abnormal blood vessels, sudden changes in blood sugar may affect the pressure or tension in the eye and possibly lead to detachment.
Myopia and Retinal Risk
Myopia, or nearsightedness, is a condition where distant objects appear blurry. It happens when the eyeball is too long or the cornea has too much curve. High myopia (severe nearsightedness) is especially important when talking about retinal problems.
In people with high myopia, the retina is stretched more than usual. This makes it thinner and more fragile. The gel-like fluid inside the eye, called the vitreous, may also pull on the retina more strongly in myopic eyes. These conditions can lead to small tears or holes in the retina, which can then lead to rhegmatogenous retinal detachment, the most common type. Myopic people are more likely to experience this, especially as they age or if they undergo eye surgeries.
Combined Risk: Myopia, Diabetes, and Semaglutide
Some individuals may have both diabetes and high myopia. This combination puts the retina at greater risk for damage. If such a person begins semaglutide treatment and experiences rapid changes in blood sugar or weight, the pressure and structure of the eye might change as well. These changes could affect how the vitreous gel interacts with the retina, increasing the chance of pulling or tearing.
It is not currently known if semaglutide directly causes retinal detachment. However, people with diabetes or myopia already have a higher chance of developing this eye problem. When these individuals begin treatment with semaglutide, especially if they have signs of diabetic retinopathy or have had previous eye problems, they may need closer monitoring.
Importance of Regular Eye Checks
For anyone with diabetes or high myopia, regular eye exams are very important. An eye doctor can check for early signs of retinal problems. If any changes are found, steps can be taken to prevent further damage. People starting semaglutide who already have eye conditions may benefit from seeing an eye specialist before beginning treatment.
Reporting new symptoms like flashes of light, a sudden increase in floaters, or a shadow in the vision is also important. These can be early signs of retinal detachment. Getting help quickly can protect vision.
People with diabetes or myopia already face higher risks for retinal detachment. Although semaglutide is helpful for blood sugar and weight control, it may need to be used carefully in these groups. More research is needed, but being aware of symptoms and keeping up with eye care can help reduce the risk of serious eye problems.
How Common is Retinal Detachment in Patients Using Semaglutide?
Retinal detachment is a serious eye condition. It happens when the retina—the thin layer of tissue at the back of the eye—pulls away from the wall of the eye. This stops the retina from getting oxygen and nutrients. If it is not treated quickly, it can lead to permanent vision loss. Some patients using semaglutide have reported vision problems, and there is growing interest in whether semaglutide can be linked to retinal detachment.
To understand how often this happens in semaglutide users, it is helpful to first look at how common retinal detachment is in general.
Baseline Risk of Retinal Detachment
In the general population, retinal detachment is considered rare. It affects about 1 in 10,000 people each year. However, some groups are at higher risk. People with severe nearsightedness (myopia), those who have had eye injuries, or those who have had eye surgery (like cataract surgery) are more likely to experience a detachment. Diabetic patients are also at higher risk because diabetes can damage the blood vessels in the retina over time. This condition is called diabetic retinopathy. If this damage is severe, it can lead to scar tissue forming, which can pull on the retina and cause a detachment.
Since semaglutide is often used in patients with type 2 diabetes and obesity—two groups already at higher risk for retinal problems—it is important to consider whether the drug itself raises the risk even more.
Known Data on Semaglutide and Retinal Detachment
As of now, there is limited evidence to show a clear link between semaglutide and retinal detachment. In clinical trials such as the SUSTAIN and STEP studies, researchers did not report high numbers of retinal detachment cases. These trials included thousands of patients and were designed to test the safety and effectiveness of semaglutide for weight loss and diabetes control. Some trials did report eye problems, especially in people with diabetic eye disease, but retinal detachment was not listed as a common or expected side effect.
Even so, reports have been submitted through post-marketing surveillance systems. These systems collect information about side effects seen in real-world use, outside of controlled trials. For example, the FDA’s Adverse Event Reporting System (FAERS) and the European Medicines Agency’s EudraVigilance system have received a small number of reports about retinal detachment in people taking semaglutide. However, these reports are rare, and they do not prove that semaglutide caused the condition. In most cases, the patients also had other risk factors, such as diabetes or prior eye conditions.
Challenges in Measuring True Risk
It is difficult to know exactly how common retinal detachment is among semaglutide users. One reason is that spontaneous reports to regulatory agencies often do not include full medical histories. It is hard to tell if the eye problem was caused by the drug or by another health condition the person already had. Also, since semaglutide is being prescribed more often, especially for weight loss, the number of people using it is growing fast. With more users, rare side effects may start to show up more clearly.
Another challenge is that people who improve their blood sugar levels quickly—especially if they had high levels for a long time—might develop or worsen eye problems. This can happen even without semaglutide. It is known as “early worsening” of diabetic retinopathy. This might explain why some patients on semaglutide experience vision changes soon after starting the medication.
Right now, retinal detachment is not listed as a common or expected side effect of semaglutide. Most studies have not shown a clear increase in risk. A few case reports suggest that it could happen, but these are rare and may involve other factors. Researchers and doctors are continuing to monitor this issue. More data from ongoing trials and real-world use will help clarify the risks over time.
Doctors should continue to watch for any visual symptoms in patients taking semaglutide, especially those with a history of eye disease. Patients who see flashes of light, a sudden increase in floaters, or a shadow across their vision should get eye care right away. Early treatment of retinal detachment can prevent vision loss.
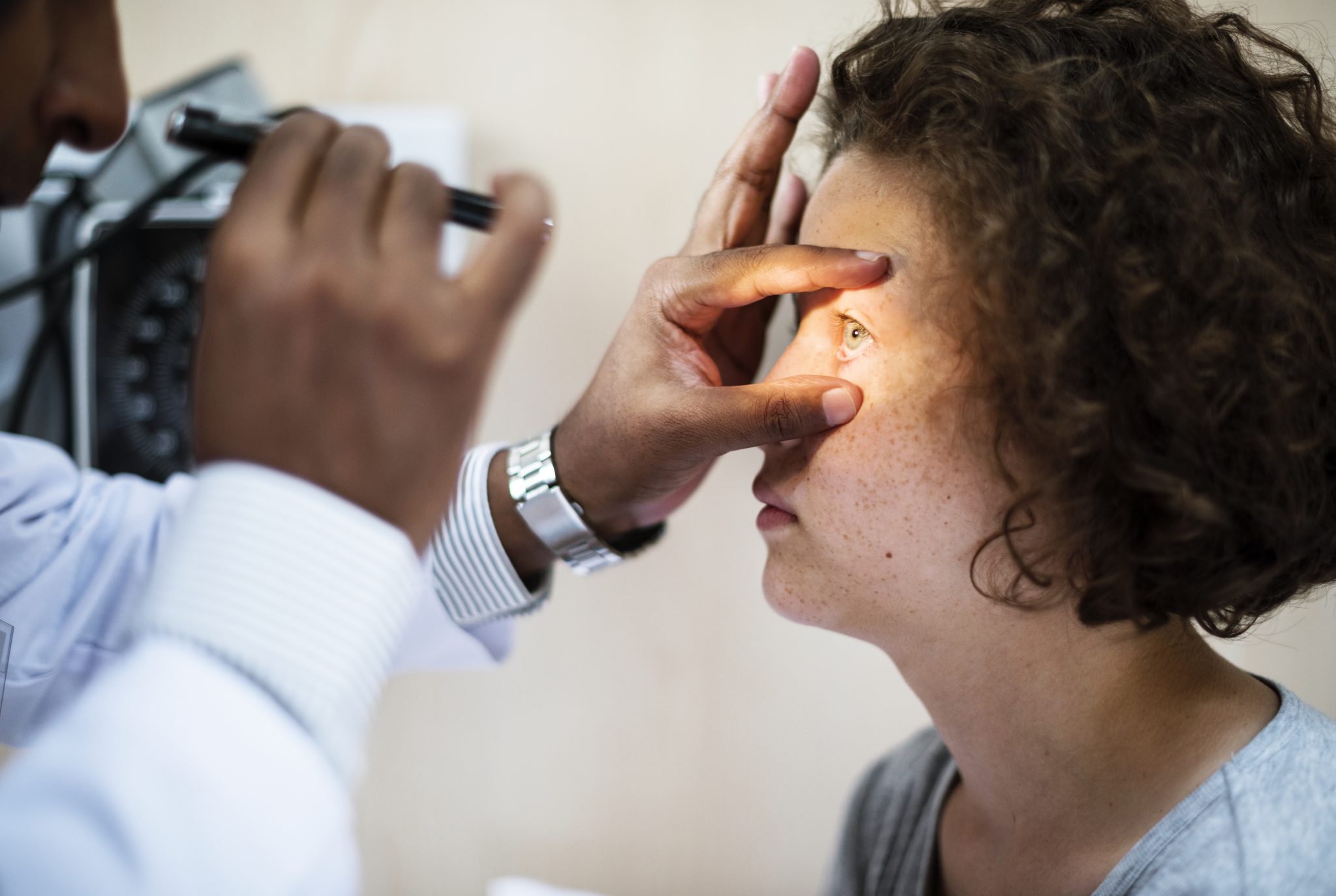
What Are Regulatory Authorities Saying About This Risk?
Health authorities around the world are responsible for monitoring the safety of medications like semaglutide. These organizations look at new research, reports from doctors and patients, and results from clinical trials. When possible risks appear—such as concerns about retinal detachment—they begin careful reviews. These reviews help protect the public and guide doctors in making informed decisions.
United States Food and Drug Administration (FDA)
The FDA keeps a close watch on medicines through a system called the Adverse Event Reporting System (FAERS). This database collects reports of side effects that may happen after using a drug. As of now, the FDA has not released a public safety alert specifically linking semaglutide to retinal detachment. However, the FDA continues to monitor reported side effects for semaglutide, including those affecting the eyes.
So far, the official labeling for semaglutide products like Ozempic, Wegovy, and Rybelsus does not include retinal detachment as a known side effect. The labels do mention other eye-related warnings, such as diabetic retinopathy complications in some patients with type 2 diabetes, especially when blood sugar levels change quickly.
The FDA often updates drug labels if enough evidence shows a real risk. These updates can include new warnings, precautions, or changes to patient instructions. For now, no such update has been made about retinal detachment and semaglutide.
European Medicines Agency (EMA)
The European Medicines Agency, or EMA, plays a similar role in the European Union. It works with a network of experts and national health agencies to evaluate the safety of medicines. Like the FDA, the EMA uses a system for collecting reports of suspected side effects. This system is called EudraVigilance.
Some reports in EudraVigilance have mentioned retinal problems in people using semaglutide, but these are mostly isolated cases. There has been no official warning or label change from the EMA regarding retinal detachment as of now. The agency continues to review incoming safety data for GLP-1 receptor agonists, including semaglutide.
The EMA has previously advised caution when using semaglutide in patients with existing diabetic eye disease. This is based on earlier studies that showed a possible worsening of diabetic retinopathy in some patients. However, no link to retinal detachment has been confirmed.
Health Canada
Health Canada also reviews and investigates reports of serious side effects. The Canadian Product Monograph for semaglutide includes information about diabetic retinopathy changes, especially when blood sugar drops quickly. However, it does not list retinal detachment as a confirmed risk.
Like other agencies, Health Canada depends on healthcare providers and the public to report side effects. These reports help signal possible problems, but they do not prove that the drug caused the event. Investigations are usually needed before label changes are made.
United Kingdom’s Medicines and Healthcare Products Regulatory Agency (MHRA)
The MHRA in the United Kingdom has similar procedures. It uses the Yellow Card Scheme to collect side effect reports from healthcare professionals and patients. The MHRA reviews this information to find possible safety signals.
As of the latest updates, the MHRA has not issued any warning or label update regarding retinal detachment with semaglutide use. The product information focuses more on common side effects like nausea and rare complications like pancreatitis or gallbladder problems.
Ongoing Safety Reviews and Monitoring
All of these agencies are committed to safety and continue to collect and analyze data. When patterns are found, such as an increase in eye problems, the agencies may:
- Start formal investigations
- Require the drug company to provide more data
- Request new studies or trials
- Update product labeling
- Issue safety communications to the public and healthcare providers
At this time, retinal detachment is not a proven side effect of semaglutide. The current evidence is limited, and no direct cause has been confirmed. However, regulatory authorities are aware of the concerns and are actively monitoring all incoming reports.
Doctors are encouraged to report any suspected side effects, including vision changes, through the appropriate national systems. These reports help agencies detect new risks early and protect patients from harm.
No major regulatory agency has confirmed a link between semaglutide and retinal detachment. There have been some reports of eye-related issues, but not enough to prove a direct connection. Agencies like the FDA, EMA, Health Canada, and MHRA continue to track safety data. If a real risk is confirmed in the future, updates will be made to labels and safety guidance. For now, patients and doctors should stay informed, especially if any changes in vision occur during treatment.
What Should Clinicians and Patients Be Aware Of?
Semaglutide is a medication used to treat type 2 diabetes and support weight loss. It works by mimicking a natural hormone in the body called GLP-1, which helps lower blood sugar and reduce appetite. While semaglutide has been helpful for many people, some reports have raised questions about a possible link between this medicine and problems with the eye, including a rare condition called retinal detachment.
Even though there is no proven connection yet, it is important for healthcare providers and patients to understand how to stay safe and what warning signs to look out for. This includes keeping an eye on visual symptoms and knowing when to seek help. There are also special steps to take for people who may be at higher risk.
Eye Checks Before Starting Semaglutide
Before beginning treatment with semaglutide, people who already have eye problems should have a full eye exam. This is especially important for those with diabetes, because diabetes can damage the small blood vessels in the back of the eye, a condition called diabetic retinopathy. High levels of blood sugar over time can lead to changes in the retina, which may make it more fragile or prone to damage.
Getting a baseline eye exam can help doctors monitor any changes over time. If a person already has signs of retinal damage, such as swelling or bleeding, doctors might need to be more cautious with how quickly blood sugar is lowered. Sudden drops in blood sugar, especially when large doses of semaglutide are used, might affect the eye in some cases, although this is still being studied.
Recognizing Warning Signs of Retinal Problems
Retinal detachment is rare, but it is a serious condition. If not treated quickly, it can lead to permanent vision loss. This makes it very important to know the early symptoms. People who are taking semaglutide should be taught to watch for the following signs:
- Sudden flashes of light in one or both eyes
- An increase in floaters, which look like tiny dark spots or squiggly lines in the vision
- A shadow or curtain that seems to move across the field of vision
- Sudden loss of part of the visual field, like losing side vision
These symptoms can happen without pain. If any of these signs appear, it is important to see an eye doctor right away. Quick treatment can often repair the retina and protect vision.
Who May Be at Higher Risk?
Some people have a greater chance of developing retinal problems, even without taking semaglutide. This includes:
- People with high levels of nearsightedness (also called high myopia)
- Those with a history of eye surgery or eye injury
- People with diabetic retinopathy
- Older adults, especially those over the age of 50
- Anyone with a family history of retinal detachment
For people in these groups, it is important for healthcare providers to discuss the possible eye risks of any new medicine. This does not mean that semaglutide should not be used, but extra care may be needed.
How Doctors Can Help Monitor for Eye Issues
Doctors who prescribe semaglutide should keep good records of any vision symptoms reported by patients. If patients describe any eye problems, they should be referred to an ophthalmologist (eye specialist) quickly. Even if the symptoms turn out to be minor, it is better to be cautious. Follow-up eye exams may be needed during treatment, especially for patients who already have diabetic eye disease.
In some cases, doctors may choose to start with a lower dose of semaglutide and increase it slowly. This may help reduce sudden changes in blood sugar that might affect the retina. Again, more research is needed to know whether this makes a difference, but it is one way to take a careful approach.
Teaching Patients to Speak Up About Vision Changes
Many people do not realize how serious retinal detachment can be. That is why education is so important. Patients should be encouraged to speak up if they notice any changes in their vision—no matter how small. A floaters or flashes of light may not seem serious at first, but these can be warning signs of a bigger problem.
Doctors, nurses, and pharmacists can all help by reminding patients to watch their vision and not delay care if something seems wrong. Eye problems should never be ignored or assumed to be normal.
By being alert and well-informed, both healthcare professionals and patients can take steps to protect vision. While the possible link between semaglutide and retinal detachment is still under study, paying attention to eye health can reduce risks and lead to better outcomes for those using this medication.
Conclusion
Semaglutide is a medication used to help manage type 2 diabetes and obesity. It works by copying the action of a natural hormone in the body called GLP-1. This hormone helps lower blood sugar and reduce appetite. Many people have seen benefits from using semaglutide. However, as with all medicines, there may be side effects. One concern that has gained attention is the possible link between semaglutide and a serious eye condition known as retinal detachment.
Retinal detachment happens when the retina pulls away from the back of the eye. This can lead to vision loss if not treated quickly. Symptoms can include sudden floaters, flashing lights, or a dark curtain over part of the vision. While retinal detachment has many known causes—such as eye injury, high levels of nearsightedness, and diabetes—there have been some reports of this condition occurring in people taking semaglutide. These reports have led researchers and doctors to look more closely at whether semaglutide could be playing a role.
So far, there is no clear proof that semaglutide directly causes retinal detachment. In clinical trials, most people taking semaglutide did not report serious eye problems. The few cases that have been mentioned in medical journals or safety databases do not show a pattern strong enough to confirm a direct link. Still, even rare events are important to study, especially when they involve a possible threat to vision.
One possible reason why this issue is being looked at is because semaglutide can cause rapid changes in blood sugar, especially in people with diabetes who are just starting the medication. A quick drop in blood sugar levels may sometimes lead to changes in the small blood vessels in the eye. In people who already have diabetic eye disease, these changes may increase the risk of eye complications. Some experts have suggested that this may be a possible way semaglutide could be linked to retinal problems, but more research is needed to confirm it.
Another idea is that semaglutide may affect other parts of the eye, such as the vitreous gel or the retina itself. GLP-1 receptors have been found in some eye tissues, but it is not fully understood how semaglutide might interact with these parts. The biological pathway is still unclear, and further studies are needed to explore how this medication might impact the structure of the eye.
People with diabetes already face a higher risk of eye problems, including retinal detachment. The same is true for people who are very nearsighted or who have had eye surgeries in the past. For these individuals, it may be more difficult to tell whether semaglutide increases their risk or whether their risk comes from other causes. That is why eye doctors and healthcare providers are advised to keep a close watch on patients with known eye conditions when they start or continue semaglutide.
So far, major health agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have not confirmed a direct link between semaglutide and retinal detachment. These organizations continue to monitor the safety of the drug and review new data as it becomes available. There have not been any major changes to the medication’s official safety information, but health authorities do encourage reporting of any unusual side effects so that patterns can be identified more quickly.
For patients who are using semaglutide, awareness is key. Any sudden changes in vision—like flashes of light, new floaters, or a dark area in the visual field—should be reported to a doctor right away. These may be signs of retinal detachment or other eye problems that need urgent care. Catching these issues early can make a big difference in treatment and recovery.
Healthcare providers are encouraged to talk to patients about their eye health, especially before starting semaglutide. An eye exam can help detect existing problems that may need follow-up during treatment. Ongoing monitoring for high-risk individuals, such as those with diabetic retinopathy, may also be a good idea, although there are no specific guidelines requiring this at the moment.
In summary, the link between semaglutide and retinal detachment is still being studied. At this time, there is no confirmed cause-and-effect relationship. The overall safety profile of semaglutide remains strong, but doctors and patients should stay alert for any eye symptoms, especially in those with a history of eye disease. Continued research, patient reporting, and careful monitoring will help provide clearer answers in the future.
Research Citations
Cool, D., Coventon, J., & Sharma, A. (2024). Semaglutide inducing resolution of proliferative diabetic retinopathy: A case report. Case Reports in Ophthalmological Medicine, 2024, Article 5834769. https://doi.org/10.1155/2024/5834769
Bloomgarden, Z. T. (2025). Semaglutide and the retina. Journal of Diabetes, 17(4), e70085. https://doi.org/10.1111/1753-0407.70085
Fadini, G. P., Sarangdhar, M., & Avogaro, A. (2018). Glucagon-like peptide-1 receptor agonists are not associated with retinal adverse events in the FDA Adverse Event Reporting System. BMJ Open Diabetes Research & Care, 6(1), e000475. https://doi.org/10.1136/bmjdrc-2017-000475
Joo, J. H., Sharma, N., Shaia, J. K., Wu, A. K., Skugor, M., Singh, R. P., & Rachitskaya, A. V. (2024). The effect of glucagon-like peptide-1 receptor agonists on diabetic retinopathy at a tertiary care center. Ophthalmology Science, 4(6), Article 100547. https://doi.org/10.1016/j.ophtha.2024.100547 (published by the American Academy of Ophthalmology)
Michaeli, T., Khateb, S., & Levy, J. (2024). The effect of glucagon-like-peptide-1 receptor agonists on diabetic retinopathy progression, central subfield thickness, and response to intravitreal injections. Journal of Clinical Medicine, 13(20), 6269. https://doi.org/10.3390/jcm13206269
Lakhani, M., et al. (2025). Association of glucagon-like peptide-1 receptor agonists with retinal and optic nerve adverse events, including retinal/vitreous detachment, in pharmacovigilance databases. American Journal of Ophthalmology. Advance online publication. https://doi.org/10.1016/j.ajo.2025.03.039 (Note: the semaglutide signal for retinal/vitreous detachment and related complications is a key finding in this study.)
Akil, H., Burgess, J., Nevitt, S., Harding, S. P., Alam, U., & Burgess, P. (2022). Early worsening of retinopathy in type 1 and type 2 diabetes after rapid improvement in glycaemic control: A systematic review. Diabetes Therapy. https://doi.org/10.1007/s13300-021-01190-z
Marso, S. P., Bain, S. C., Consoli, A., et al. (2016). Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. The New England Journal of Medicine, 375(19), 1834–1844. https://doi.org/10.1056/NEJMoa1607141 (Includes the SUSTAIN-6 data signal of retinopathy worsening, which underpins later concerns about tractional complications.)
Vujosevic, S., Toma, C., Ferrulli, A., De Cillà, S., Nucci, P., & Luzi, L. (2025). New generation agents for glycemic control and diabetic retinopathy progression: What we need to know? Acta Diabetologica. Advance online publication. https://doi.org/10.1007/s00592-025-02552-w
Bain, S. C., Klufas, M. A., Ho, A., & Matthews, D. R. (2019). Worsening of diabetic retinopathy with rapid improvement in systemic glucose control: A review. Diabetes, Obesity and Metabolism, 21(3), 454–466. https://doi.org/10.1111/dom.13511
Questions and Answers: Semaglutide and Retinal Detachment
Semaglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist used primarily to manage type 2 diabetes and for weight loss in certain patients.
Retinal detachment is a serious eye condition where the retina pulls away from its normal position, which can lead to permanent vision loss if not treated promptly.
As of now, there is no established direct causal link between semaglutide and retinal detachment, but research is ongoing regarding its ocular effects, especially in diabetic patients.
Yes, some patients have reported diabetic retinopathy complications with semaglutide, particularly those with pre-existing eye disease. These are not the same as retinal detachment but are still significant.
Rapid improvement in blood glucose levels—sometimes seen with semaglutide—can temporarily worsen diabetic retinopathy, a known risk factor for retinal complications.
There is no blanket contraindication, but patients with a history of retinal disease should be monitored closely and consult an ophthalmologist before starting semaglutide.
Symptoms include sudden flashes of light, floaters, a shadow or curtain over vision, and a sudden decrease in vision—these require immediate medical attention.
As of now, the FDA has not issued any specific warnings about retinal detachment related to semaglutide, though retinopathy warnings are included in prescribing information.
Regular eye exams, gradual glucose control, and coordination between their endocrinologist and ophthalmologist can help reduce risk.
Possibly. The choice of diabetes or weight-loss medication should be personalized. Drugs with a more gradual effect on glucose levels or with less known ocular impact may be preferred in high-risk individuals.

Dr. Jay Flottman
Dr. Jay Flottmann is a physician in Panama City, FL. He received his medical degree from University of Texas Medical Branch and has been in practice 21 years. He is experienced in military medicine, an FAA medical examiner, human performance expert, and fighter pilot.
Professionally, I am a medical doctor (M.D. from the University of Texas Medical Branch at Galveston), a fighter pilot (United States Air Force trained – F-15C/F-22/AT-38C), and entrepreneur.
