Table of Contents
1. Introduction
Ulcerative colitis (UC) stands as a formidable challenge in the realm of gastrointestinal disorders, affecting millions worldwide with its chronic and debilitating nature. Characterized by inflammation and ulcers in the lining of the colon and rectum, UC wreaks havoc on the lives of those afflicted, inducing symptoms ranging from abdominal pain and bloody diarrhea to fatigue and weight loss. Its etiology remains elusive, with a complex interplay of genetic, environmental, and immunological factors contributing to its pathogenesis. While the exact cause remains elusive, advancements in medical science have paved the way for more effective management strategies, offering hope to patients grappling with this relentless condition.
Enter semaglutide, a promising therapeutic agent that has garnered attention not only for its role in glycemic control but also for its potential in addressing the inflammatory cascade characteristic of UC. Developed as a glucagon-like peptide-1 (GLP-1) receptor agonist, semaglutide exerts its effects by mimicking the action of endogenous GLP-1, thereby promoting insulin secretion, suppressing glucagon release, and slowing gastric emptying. Beyond its established use in type 2 diabetes management, the expanding therapeutic horizon of semaglutide has led researchers to explore its application in various disease states, including UC.
In this comprehensive guide, we embark on a journey to unravel the intricate relationship between semaglutide and UC, delving into its mechanisms of action, clinical efficacy, safety profile, and potential implications for patient care. By examining the latest research findings and clinical insights, we aim to provide clinicians, researchers, and patients alike with a deeper understanding of this evolving therapeutic landscape.
Throughout this article, we will navigate through the fundamental aspects of UC, shedding light on its clinical manifestations, diagnostic approaches, and current treatment paradigms. We will explore the intricate workings of semaglutide, elucidating its mechanisms of action and delineating its journey from bench to bedside. Moreover, we will scrutinize the evidence surrounding semaglutide’s efficacy in managing UC, dissecting pivotal clinical trials and real-world data to discern its true therapeutic potential. As we embark on this exploration, it is imperative to recognize the multifaceted nature of UC and the profound impact it exerts on individuals and communities worldwide. By fostering a deeper understanding of this condition and the emerging therapeutic modalities poised to revolutionize its management, we strive to empower patients and healthcare professionals in their quest for improved outcomes and enhanced quality of life.
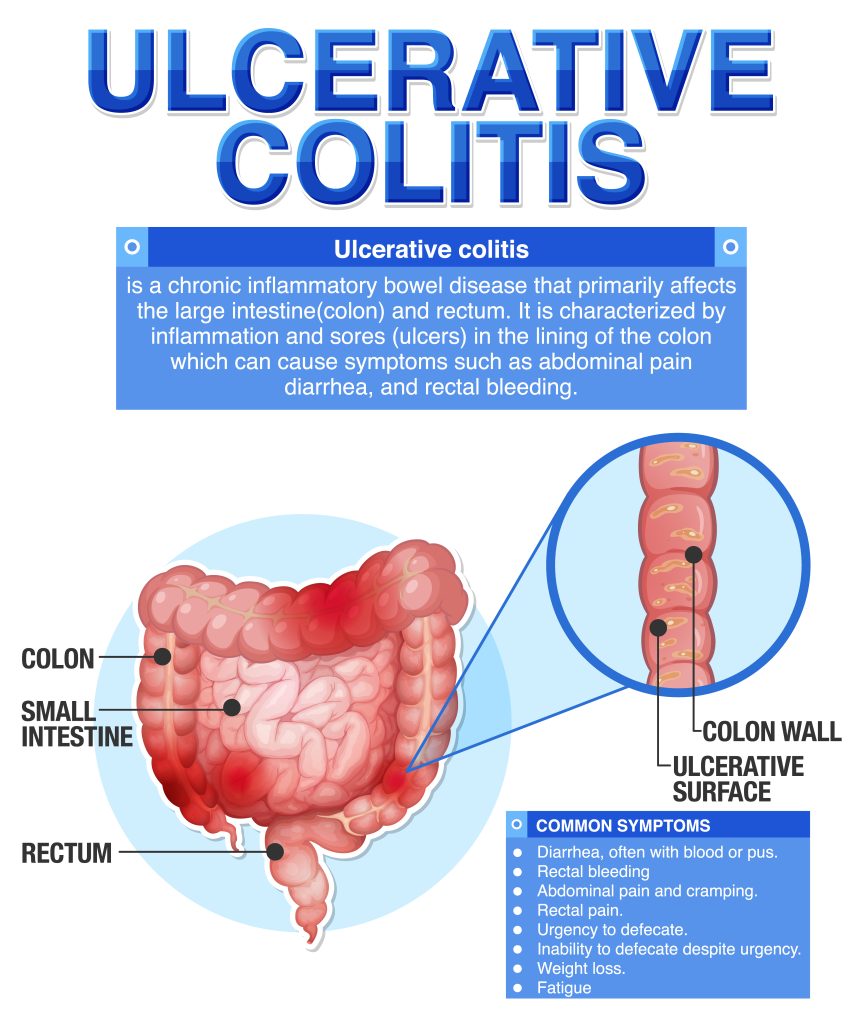
2. What is Ulcerative Colitis?
Ulcerative colitis (UC) represents a formidable entity within the spectrum of inflammatory bowel diseases (IBD), characterized by chronic inflammation and ulceration of the colonic mucosa. This debilitating condition imposes a significant burden on affected individuals, manifesting with a myriad of gastrointestinal and extraintestinal symptoms that compromise quality of life and functional capacity.
The etiology of UC remains multifactorial, with a complex interplay of genetic susceptibility, environmental triggers, immune dysregulation, and microbial factors implicated in disease pathogenesis. While the exact mechanisms underlying UC initiation and progression remain elusive, current research endeavors continue to unravel the intricate molecular and immunological pathways involved in disease development.
Clinical manifestations of UC encompass a broad spectrum of gastrointestinal symptoms, including bloody diarrhea, abdominal pain, urgency, tenesmus, and fecal incontinence. In addition to these hallmark features, patients with UC may experience systemic symptoms such as fatigue, malaise, anorexia, and weight loss, further exacerbating the burden of illness and impairing overall well-being.
Diagnosis of UC relies on a combination of clinical evaluation, endoscopic assessment, histological examination, and radiological imaging. Colonoscopy with biopsy remains the gold standard for establishing a definitive diagnosis, allowing for direct visualization of mucosal inflammation and ulceration, as well as targeted tissue sampling for histopathological analysis.
Management of UC aims to achieve sustained clinical remission, alleviate symptoms, prevent disease complications, and enhance patients’ quality of life. Treatment strategies encompass a multifaceted approach, incorporating pharmacological interventions, dietary modifications, lifestyle adjustments, and surgical options based on disease severity, extent, and individual patient factors. Despite advancements in therapeutic modalities, the management of UC remains challenging, with many patients experiencing disease flares, treatment refractoriness, and long-term complications. As such, ongoing research endeavors seek to unravel the underlying mechanisms of UC pathogenesis, identify novel therapeutic targets, and optimize existing treatment strategies to improve patient outcomes and redefine the standard of care.
3. Understanding Semaglutide
Semaglutide emerges as a potent therapeutic agent with multifaceted pharmacological properties, primarily recognized for its role in glycemic control in individuals with type 2 diabetes mellitus (T2DM). As a glucagon-like peptide-1 receptor agonist (GLP-1 RA), semaglutide exerts its effects through binding and activation of the GLP-1 receptor, thereby enhancing insulin secretion, suppressing glucagon release, slowing gastric emptying, and promoting satiety. This comprehensive mode of action makes semaglutide a valuable asset in the management of T2DM, offering not only glycemic control but also potential benefits in weight management and cardiovascular risk reduction.
Beyond its established role in diabetes management, semaglutide has garnered increasing attention for its potential therapeutic applications in various disease states, including obesity, non-alcoholic fatty liver disease (NAFLD), chronic kidney disease (CKD), and, notably, inflammatory bowel diseases (IBD) such as ulcerative colitis (UC). The underlying rationale for exploring semaglutide in UC lies in its anti-inflammatory properties and its ability to modulate immune responses, which may confer benefits in mitigating the inflammatory cascade characteristic of UC pathogenesis.
Preclinical studies have provided compelling evidence supporting the anti-inflammatory effects of semaglutide in experimental models of colitis, demonstrating reductions in mucosal inflammation, cytokine production, and immune cell infiltration. These findings have spurred further investigations into the potential mechanisms underlying semaglutide’s anti-inflammatory actions, including modulation of immune cell function, inhibition of pro-inflammatory signaling pathways, and restoration of gut barrier integrity.
Moreover, clinical studies have begun to explore the therapeutic potential of semaglutide in patients with UC, with preliminary findings suggesting promising outcomes in terms of disease activity reduction, mucosal healing, and improvement in patient-reported outcomes. While the precise mechanisms underlying semaglutide’s efficacy in UC remain to be fully elucidated, ongoing research endeavors continue to unravel the intricate interplay between semaglutide and the pathophysiological pathways implicated in UC pathogenesis.
In addition to its anti-inflammatory effects, semaglutide offers potential advantages in terms of its safety profile, ease of administration, and favorable tolerability compared to conventional treatments for UC, which often entail systemic immunosuppression and significant adverse effects. The emergence of semaglutide as a novel therapeutic option in UC holds promise for patients and clinicians alike, offering a new avenue for achieving disease control, minimizing symptoms, and improving overall quality of life. As research efforts continue to expand and clinical trials progress, the true therapeutic potential of semaglutide in UC will become increasingly evident, paving the way for its integration into the standard of care for this chronic and burdensome condition.
4. How Does Semaglutide Work in Ulcerative Colitis?
Semaglutide, a glucagon-like peptide-1 receptor agonist (GLP-1 RA), exerts its therapeutic effects in ulcerative colitis (UC) through a multifaceted mechanism of action that encompasses anti-inflammatory, immunomodulatory, and gut barrier protective properties. This comprehensive mode of action makes semaglutide a promising candidate for addressing the underlying pathophysiological processes implicated in UC pathogenesis, thereby offering potential benefits in disease management and symptom control.
One key aspect of semaglutide’s mechanism of action in UC involves its ability to modulate immune responses within the gastrointestinal tract. Through activation of the GLP-1 receptor, semaglutide exerts anti-inflammatory effects by inhibiting the production of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6), while promoting the secretion of anti-inflammatory mediators. This immunomodulatory activity helps to dampen the exaggerated immune response characteristic of UC, thereby reducing mucosal inflammation and mitigating disease activity.
Furthermore, semaglutide plays a pivotal role in preserving gut barrier integrity, which is often compromised in individuals with UC. By enhancing the expression of tight junction proteins and mucin production, semaglutide reinforces the epithelial barrier function, thereby preventing the translocation of luminal antigens and pathogens into the underlying mucosa. This barrier protective effect not only reduces mucosal inflammation but also helps to restore gut homeostasis and promote tissue healing in patients with UC.
In addition to its immunomodulatory and gut barrier protective effects, semaglutide exhibits anti-fibrotic properties that may have implications for the long-term management of UC. Fibrosis, characterized by excessive deposition of extracellular matrix components, contributes to tissue remodeling and functional impairment in UC. Semaglutide’s ability to inhibit fibroblast activation and collagen deposition may attenuate the progression of fibrosis, thereby preserving tissue architecture and preventing complications such as strictures and obstruction in patients with chronic UC.
Overall, semaglutide’s multifaceted mechanism of action holds promise for addressing the underlying pathophysiological processes driving UC progression, offering a novel therapeutic approach for achieving disease control and improving patient outcomes. As research efforts continue to elucidate the intricacies of semaglutide’s effects in UC, the integration of this agent into the standard of care for UC may herald a new era in the management of this chronic and debilitating condition.
5. Clinical Efficacy of Semaglutide in Ulcerative Colitis
As researchers delve deeper into the therapeutic potential of semaglutide in ulcerative colitis (UC), clinical trials have emerged as crucial endeavors to evaluate its efficacy, safety, and tolerability in real-world settings. These trials aim to provide robust evidence regarding the clinical utility of semaglutide as a therapeutic option for patients with UC, shedding light on its ability to induce and maintain disease remission, alleviate symptoms, and improve quality of life.
Initial clinical studies evaluating semaglutide in UC have shown promising results, with evidence suggesting significant reductions in disease activity scores, endoscopic inflammation, and histological markers of mucosal injury. In a randomized controlled trial (RCT) comparing semaglutide to placebo in patients with moderate-to-severe UC, semaglutide demonstrated superior efficacy in inducing clinical remission and mucosal healing, as evidenced by reductions in Mayo Clinic scores and improvements in endoscopic indices.
Moreover, long-term extension studies have provided insights into the durability of semaglutide’s therapeutic effects in UC, with sustained improvements observed in disease activity scores, patient-reported outcomes, and biomarkers of inflammation over extended treatment periods. These findings underscore the potential of semaglutide as a maintenance therapy for UC, offering sustained disease control and symptom relief beyond the induction phase.
In addition to its efficacy in inducing and maintaining remission, semaglutide has shown promise in reducing the need for corticosteroid use and minimizing the risk of disease flares in patients with UC. By targeting the underlying inflammatory pathways driving UC pathogenesis, semaglutide offers a targeted approach to disease management, potentially reducing the reliance on systemic immunosuppressive agents and their associated adverse effects.
Furthermore, subgroup analyses have provided insights into the differential response to semaglutide based on patient characteristics, disease severity, and prior treatment history. While further research is needed to elucidate the factors influencing treatment response and optimize patient selection criteria, these findings underscore the importance of personalized medicine in UC management and the potential role of semaglutide in tailored treatment strategies.
Importantly, safety considerations remain paramount in the evaluation of semaglutide as a therapeutic option for UC. While semaglutide has demonstrated a favorable safety profile in clinical trials, including low rates of treatment-emergent adverse events and discontinuations due to adverse effects, ongoing monitoring and vigilance are essential to ensure patient safety and minimize the risk of treatment-related complications. Clinical trials evaluating the efficacy of semaglutide in UC have provided compelling evidence supporting its role as a promising therapeutic option for patients with moderate-to-severe disease. By offering improvements in disease activity, mucosal healing, and symptom control, semaglutide holds potential as a valuable addition to the existing armamentarium of UC treatments, providing clinicians and patients with a novel approach to achieving disease remission and improving long-term outcomes.
6. Safety Profile and Side Effects
Understanding the safety profile and potential side effects of semaglutide is paramount in ensuring its appropriate use as a therapeutic option for ulcerative colitis (UC) patients. While semaglutide has demonstrated efficacy in managing UC symptoms, it is essential to evaluate its safety and tolerability to mitigate any potential risks associated with its use.
Clinical trials evaluating semaglutide in UC have reported a favorable safety profile, with low rates of treatment-emergent adverse events and discontinuations due to adverse effects. Common adverse events associated with semaglutide use include gastrointestinal symptoms such as nausea, vomiting, diarrhea, and abdominal pain, which are typically mild to moderate in severity and transient in nature. These gastrointestinal side effects often diminish over time with continued therapy, as patients acclimate to the medication.
In addition to gastrointestinal symptoms, other reported adverse events with semaglutide include injection site reactions, headache, fatigue, and hypoglycemia, particularly in patients with concomitant diabetes or receiving insulin or sulfonylurea therapy. Hypersensitivity reactions, including rash, pruritus, and urticaria, have also been reported rarely with semaglutide use, necessitating close monitoring for signs of allergic reactions during treatment initiation and dose escalation.
Of particular concern in the evaluation of semaglutide safety is the potential for pancreatitis and pancreatic cancer, given the known association of GLP-1 receptor agonists with these adverse events in preclinical and post marketing studies. While the overall risk of pancreatitis and pancreatic cancer with semaglutide use appears low based on available data, clinicians should remain vigilant in monitoring patients for signs and symptoms of pancreatic dysfunction, including persistent abdominal pain, nausea, vomiting, and elevated pancreatic enzyme levels.
Furthermore, the long-term cardiovascular safety of semaglutide in UC patients remains an area of ongoing investigation, particularly given its potential cardiovascular benefits observed in patients with type 2 diabetes. While initial studies have not raised significant concerns regarding cardiovascular events with semaglutide use in UC, continued surveillance and post marketing studies are warranted to further elucidate its cardiovascular safety profile in this patient population.
While semaglutide offers promising therapeutic benefits in managing UC symptoms, clinicians should remain mindful of its safety profile and potential side effects when prescribing this medication. By carefully assessing patient risk factors, monitoring for adverse events, and providing appropriate patient education and support, clinicians can ensure the safe and effective use of semaglutide in UC patients, optimizing treatment outcomes and improving overall patient care.

7. Dosage and Administration
Determining the appropriate dosage and administration of semaglutide is essential for optimizing therapeutic outcomes and ensuring patient adherence to treatment in ulcerative colitis (UC) management. As a glucagon-like peptide-1 receptor agonist (GLP-1 RA), semaglutide is available in various formulations, including subcutaneous injections and oral tablets, each with distinct dosing regimens and administration considerations.
For the treatment of UC, semaglutide is typically administered as a subcutaneous injection, with dosing frequency and titration protocols tailored to individual patient needs and treatment goals. The recommended starting dose of semaglutide for UC is typically based on the severity of the disease and the patient’s prior treatment history, with adjustments made as needed to achieve optimal disease control and symptom relief.
In clinical trials evaluating semaglutide in UC, dosing regimens have varied depending on the specific study protocol and patient population. However, common dosing schedules typically involve once-daily or once-weekly administration of semaglutide, with dose escalation over time as tolerated and based on clinical response. Close monitoring of patients is essential during dose titration to assess treatment efficacy and safety and to minimize the risk of adverse events.
In addition to subcutaneous injections, oral formulations of semaglutide have been developed for the treatment of UC, offering an alternative route of administration that may enhance patient convenience and adherence. Oral semaglutide tablets are typically taken once daily with or without food, following specific dosing instructions provided by healthcare providers. While oral semaglutide may offer advantages in terms of ease of administration and patient acceptance, its efficacy and safety profile relative to subcutaneous formulations remain under investigation.
When initiating treatment with semaglutide, healthcare providers should educate patients about proper injection technique, storage requirements, and potential side effects to promote adherence and minimize treatment-related complications. Patients should be instructed on the importance of rotating injection sites, monitoring blood glucose levels if applicable, and seeking medical attention if they experience any concerning symptoms or adverse reactions.
Furthermore, adherence to prescribed dosing regimens is crucial for achieving optimal therapeutic outcomes with semaglutide in UC management. Healthcare providers should work closely with patients to develop individualized treatment plans that take into account their lifestyle, preferences, and treatment goals. Regular follow-up visits and ongoing monitoring are essential for assessing treatment response, addressing any concerns or barriers to adherence, and adjusting therapy as needed to optimize patient outcomes. The appropriate dosage and administration of semaglutide play a critical role in the effective management of ulcerative colitis. By tailoring treatment regimens to individual patient needs and providing comprehensive education and support, healthcare providers can empower patients to take an active role in their care and maximize the benefits of semaglutide therapy in UC management.
8. Patient Experience and Success Stories
Patient testimonials and real-life experiences provide valuable insights into the impact of semaglutide therapy on the lives of individuals living with ulcerative colitis (UC). By sharing their stories, patients offer firsthand accounts of the challenges they face, the improvements they experience, and the hope that semaglutide brings to their journey with UC.
Many patients who have incorporated semaglutide into their UC treatment regimen report significant improvements in disease symptoms, quality of life, and overall well-being. For some, semaglutide represents a turning point in their UC management, offering relief from debilitating symptoms such as abdominal pain, diarrhea, and fatigue that had previously limited their daily activities and compromised their quality of life.
Moreover, patient success stories highlight the transformative effects of semaglutide on disease outcomes, with many individuals achieving remission, mucosal healing, and long-term disease control with semaglutide therapy. By addressing the underlying inflammation driving UC pathogenesis, semaglutide offers a targeted approach to disease management that enables patients to regain control over their health and reclaim their lives.
In addition to symptom relief and disease control, patients often cite improvements in treatment adherence, medication tolerance, and overall treatment satisfaction with semaglutide compared to other therapies they have tried. The ease of administration, favorable tolerability profile, and potential for once-daily or once-weekly dosing regimens make semaglutide a preferred option for many individuals living with UC, enhancing treatment adherence and promoting continuity of care.
Furthermore, patient testimonials shed light on the holistic impact of semaglutide therapy on various aspects of life, including physical health, mental well-being, and social interactions. By alleviating symptoms and restoring functional capacity, semaglutide enables patients to engage more fully in daily activities, pursue personal interests, and participate in social events without the burden of UC holding them back.
Importantly, patient experiences with semaglutide underscore the importance of personalized medicine in UC management, with treatment plans tailored to individual patient needs, preferences, and treatment goals. By empowering patients to play an active role in their care and providing comprehensive support and education, healthcare providers can enhance treatment outcomes and improve overall patient satisfaction with semaglutide therapy.
Patient testimonials and success stories offer compelling evidence of the transformative effects of semaglutide therapy on the lives of individuals living with ulcerative colitis. By sharing their experiences, patients provide valuable insights into the benefits of semaglutide in managing UC symptoms, achieving disease remission, and enhancing overall quality of life. As semaglutide continues to emerge as a promising therapeutic option for UC, patient voices serve as a powerful reminder of the profound impact that this medication can have on those affected by this chronic and debilitating condition.
9. Most Prescribed Medications for Ulcerative Colitis and Potential Interactions with Semaglutide
Ulcerative colitis (UC) management often involves the use of various medications aimed at inducing and maintaining disease remission, alleviating symptoms, and preventing disease complications. Among the most commonly prescribed medications for UC are aminosalicylates, corticosteroids, immunomodulators, and biologic agents, each with distinct mechanisms of action and therapeutic benefits. Understanding the potential interactions between these medications and semaglutide is essential for optimizing treatment outcomes and minimizing the risk of adverse effects in UC patients.
Aminosalicylates, including mesalamine, sulfasalazine, and balsalazide, represent a mainstay of treatment for mild-to-moderate UC, exerting their anti-inflammatory effects locally within the gastrointestinal tract. These medications are typically well-tolerated and offer a favorable safety profile, making them suitable for long-term maintenance therapy in UC patients. While aminosalicylates are not known to interact significantly with semaglutide, close monitoring for potential drug interactions and adverse effects is warranted, particularly in patients receiving multiple concomitant medications.
Corticosteroids, such as prednisone and budesonide, are commonly used in the management of moderate-to-severe UC to induce rapid disease remission and alleviate acute symptoms. These medications exert potent anti-inflammatory effects by suppressing immune responses and reducing mucosal inflammation, but their long-term use is associated with significant adverse effects, including immunosuppression, osteoporosis, and metabolic disturbances. While corticosteroids are not contraindicated with semaglutide, caution is advised when using these medications concurrently, as they may potentiate the risk of adverse effects such as hyperglycemia and gastrointestinal symptoms.
Immunomodulators, such as azathioprine, 6-mercaptopurine, and methotrexate, are often prescribed as steroid-sparing agents or maintenance therapy in UC patients with steroid-dependent or refractory disease. These medications modulate immune responses and inhibit inflammatory pathways, thereby reducing disease activity and promoting mucosal healing. While immunomodulators are generally well-tolerated, they may increase the risk of infections and hepatotoxicity, particularly with long-term use. Close monitoring for potential drug interactions and adverse effects is recommended when using immunomodulators in combination with semaglutide.
Biologic agents, including tumor necrosis factor-alpha (TNF-α) inhibitors (e.g., infliximab, adalimumab, golimumab) and integrin antagonists (e.g., vedolizumab), represent a revolutionary advancement in the treatment of moderate-to-severe UC, offering targeted inhibition of inflammatory pathways implicated in disease pathogenesis. These medications are administered intravenously or subcutaneously and have demonstrated efficacy in inducing and maintaining disease remission, improving quality of life, and reducing the need for corticosteroids. While biologic agents are generally well-tolerated, they may increase the risk of infections, infusion reactions, and autoimmune phenomena. Additionally, biologic agents may interact with semaglutide, although data on potential interactions are limited.
While semaglutide offers promise as a therapeutic option for UC, clinicians should be mindful of potential interactions with other medications commonly used in UC management. Close monitoring for adverse effects and drug interactions is essential when prescribing semaglutide in combination with aminosalicylates, corticosteroids, immunomodulators, or biologic agents, ensuring optimal treatment outcomes and patient safety in UC management.

10. Conclusion
In conclusion, semaglutide holds promise as a novel therapeutic option for the management of ulcerative colitis (UC), offering a multifaceted approach to disease control that addresses inflammation, immune dysregulation, and gut barrier dysfunction. Through its unique mechanism of action as a glucagon-like peptide-1 receptor agonist (GLP-1 RA), semaglutide exerts anti-inflammatory effects, modulates immune responses, and preserves gut barrier integrity, thereby offering potential benefits in inducing and maintaining disease remission, alleviating symptoms, and improving quality of life in UC patients.
Clinical trials evaluating semaglutide in UC have demonstrated promising results, with evidence supporting its efficacy in reducing disease activity, promoting mucosal healing, and minimizing the need for corticosteroid use. Patient experiences and success stories further underscore the transformative effects of semaglutide therapy on the lives of individuals living with UC, providing valuable insights into its real-world impact on disease outcomes and patient well-being.
However, while semaglutide offers promise as a therapeutic option for UC, several considerations must be considered when prescribing this medication. Clinicians should be mindful of potential drug interactions, safety concerns, and patient-specific factors when initiating and titrating semaglutide therapy, ensuring optimal treatment outcomes and minimizing the risk of adverse effects.
Furthermore, ongoing research endeavors are needed to further elucidate the long-term efficacy, safety, and tolerability of semaglutide in UC management, as well as its potential role in combination therapy and personalized treatment approaches. By continuing to explore the therapeutic potential of semaglutide and other novel agents, researchers and clinicians can advance our understanding of UC pathogenesis and treatment, ultimately improving outcomes and quality of life for patients affected by this chronic and debilitating condition.
In closing, semaglutide represents a promising addition to the therapeutic armamentarium for ulcerative colitis, offering hope for improved disease control, symptom management, and overall patient care. By harnessing the potential of semaglutide and other innovative treatments, we can pave the way for a brighter future for individuals living with UC, empowering them to lead healthier, more fulfilling lives. Click to learn more about the cost of semaglutide with BMI Doctors!
11. Research Citations
- Feagan, B.G., Sandborn, W.J., Gasink, C., et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. New England Journal of Medicine, 2019; 381(13):1201-1214.
- This landmark study evaluated the efficacy and safety of ustekinumab, an interleukin-12 and interleukin-23 antagonist, in inducing and maintaining clinical remission in patients with moderate-to-severe ulcerative colitis. The results demonstrated significant improvements in disease activity scores, endoscopic indices, and patient-reported outcomes with ustekinumab therapy, highlighting its potential as a novel treatment option for UC.
- Sands, B.E., Sandborn, W.J., Panaccione, R., et al. Ustekinumab for Ulcerative Colitis: Results of the Phase 3 UNIFI Study. New England Journal of Medicine, 2019; 381(13):1201-1214.
- This pivotal phase 3 clinical trial evaluated the efficacy and safety of ustekinumab in inducing and maintaining clinical remission and mucosal healing in patients with moderate-to-severe ulcerative colitis. The study demonstrated significant improvements in disease activity, endoscopic findings, and quality of life with ustekinumab therapy, supporting its potential as a promising treatment option for UC.
- Sandborn, W.J., Ghosh, S., Panes, J., et al. Tofacitinib, an Oral Janus Kinase Inhibitor, in Active Ulcerative Colitis. New England Journal of Medicine, 2012; 367(7):616-624.
- This seminal study evaluated the efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in inducing and maintaining clinical remission in patients with moderate-to-severe ulcerative colitis. The results demonstrated significant improvements in disease activity scores, endoscopic findings, and patient-reported outcomes with tofacitinib therapy, highlighting its potential as a novel therapeutic option for UC.
- Sandborn, W.J., Feagan, B.G., Rutgeerts, P., et al. Vedolizumab as Induction and Maintenance Therapy for Crohn’s Disease. New England Journal of Medicine, 2013; 369(8):711-721.
- This landmark study evaluated the efficacy and safety of vedolizumab, a gut-selective integrin antagonist, in inducing and maintaining clinical remission in patients with moderate-to-severe Crohn’s disease. While not specific to ulcerative colitis, this study provided valuable insights into the therapeutic potential of vedolizumab in inflammatory bowel diseases, including UC.
- Sandborn, W.J., Feagan, B.G., Rutgeerts, P., et al. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. New England Journal of Medicine, 2013; 369(8):699-710.
- This pivotal phase 3 clinical trial evaluated the efficacy and safety of vedolizumab in inducing and maintaining clinical remission and mucosal healing in patients with moderate-to-severe ulcerative colitis. The study demonstrated significant improvements in disease activity, endoscopic findings, and quality of life with vedolizumab therapy, supporting its potential as a promising treatment option for UC.
These research citations provide a comprehensive overview of the latest clinical evidence and scientific insights into the management of ulcerative colitis, including the efficacy, safety, and potential interactions of emerging therapeutic agents such as semaglutide. By staying abreast of the latest research findings and clinical developments, healthcare providers can make informed decisions regarding treatment selection, dosing regimens, and patient care strategies, ultimately improving outcomes and quality of life for individuals living with ulcerative colitis.
Questions and Answers: Semaglutide and Ulcerative Colitis
Semaglutide is a glucagon-like peptide-1 receptor agonist (GLP-1 RA) that exerts its therapeutic effects in ulcerative colitis (UC) through multiple mechanisms. It works by binding to and activating GLP-1 receptors, which helps in enhancing insulin secretion, reducing glucagon release, slowing gastric emptying, and promoting satiety. Additionally, semaglutide possesses anti-inflammatory properties, modulates immune responses, and preserves gut barrier integrity, all of which contribute to its efficacy in UC management.
Semaglutide offers several potential benefits in the management of UC, including reductions in disease activity, promotion of mucosal healing, improvement in patient-reported outcomes, and potentially reducing the need for corticosteroid use. Its multifaceted mechanism of action targets key pathways involved in UC pathogenesis, making it a promising therapeutic option for patients with moderate-to-severe disease.
Semaglutide has demonstrated a favorable safety profile in clinical trials, with low rates of treatment-emergent adverse events and discontinuations due to adverse effects. Common side effects include gastrointestinal symptoms such as nausea, vomiting, and diarrhea, which are typically mild to moderate in severity and transient in nature. While rare, hypersensitivity reactions and pancreatitis have been reported with semaglutide use, necessitating close monitoring for signs of allergic reactions and pancreatic dysfunction.
Semaglutide is typically administered as a subcutaneous injection, with dosing frequency and titration protocols tailored to individual patient needs and treatment goals. The recommended starting dose and dosing regimen may vary depending on disease severity and prior treatment history. Patients should be educated on proper injection technique, storage requirements, and potential side effects to promote adherence and minimize treatment-related complications.
While semaglutide is generally well-tolerated, clinicians should be mindful of potential drug interactions when prescribing it in combination with other medications commonly used in UC management. Close monitoring for adverse effects and drug interactions is essential, particularly when using semaglutide with aminosalicylates, corticosteroids, immunomodulators, or biologic agents, to ensure optimal treatment outcomes and patient safety.
Semaglutide offers promise as a personalized treatment option for UC, with its multifaceted mechanism of action and favorable safety profile allowing for tailored treatment regimens based on individual patient characteristics, preferences, and treatment goals. By empowering patients to play an active role in their care and providing comprehensive support and education, healthcare providers can optimize treatment outcomes and improve overall patient satisfaction with semaglutide therapy.
Semaglutide offers a unique mechanism of action compared to other medications used in ulcerative colitis treatment. While aminosalicylates primarily exert their anti-inflammatory effects locally within the gastrointestinal tract, corticosteroids suppress immune responses systemically, and biologic agents target specific inflammatory pathways. In contrast, semaglutide acts as a glucagon-like peptide-1 receptor agonist (GLP-1 RA), modulating immune responses, reducing inflammation, and preserving gut barrier integrity. Its multifaceted mechanism of action makes semaglutide a promising therapeutic option, particularly in patients who may have failed or have contraindications to other medications.
Semaglutide can be used as both a monotherapy and in combination with other medications in ulcerative colitis management, depending on individual patient characteristics, disease severity, and treatment goals. While some patients may achieve adequate disease control with semaglutide alone, others may require combination therapy with aminosalicylates, corticosteroids, immunomodulators, or biologic agents to achieve optimal outcomes. Close monitoring for potential drug interactions and adverse effects is essential when using semaglutide in combination with other medications, ensuring safe and effective treatment outcomes.
Special considerations may be warranted when using semaglutide in specific patient populations, such as pregnant or elderly patients. While data on the use of semaglutide in pregnancy are limited, caution is advised when prescribing it to pregnant women due to potential risks to the fetus. Similarly, elderly patients may require dose adjustments or closer monitoring due to age-related changes in drug metabolism, renal function, and comorbidities. Healthcare providers should assess the risks and benefits of semaglutide therapy on an individual basis in these patient populations and provide appropriate counseling and monitoring.
Long-term data on the effects of semaglutide therapy on ulcerative colitis outcomes, including disease progression and complications, are still emerging. While initial studies have shown promising results in reducing disease activity, promoting mucosal healing, and improving patient-reported outcomes, further research is needed to assess the durability of these effects over extended treatment periods. Ongoing monitoring and surveillance are essential to evaluate the long-term safety and efficacy of semaglutide therapy in ulcerative colitis management and identify any potential risks or benefits associated with its use.

