Table of Contents
Introduction
Tirzepatide is a prescription medicine that has become widely known in recent years. It is a type of medication called a GLP-1 and GIP receptor agonist, which means it works on two hormones in the body that help control blood sugar and appetite. The U.S. Food and Drug Administration (FDA) has approved tirzepatide for the treatment of type 2 diabetes under the brand name Mounjaro®. It is also available under the brand name Zepbound® for chronic weight management in people who meet certain health criteria. Both products are made by the pharmaceutical company Eli Lilly.
Because tirzepatide helps lower blood sugar, reduce body weight, and improve overall metabolic health, many patients are interested in it. Providers have also shown strong interest because it offers a new tool for treating obesity and diabetes, two conditions that affect millions of people in the United States. However, as more people look for this medicine, one important question comes up again and again: Can tirzepatide be compounded?
Before answering that question, it is helpful to explain what “compounding” means. In pharmacy practice, compounding is the process of creating a medication by mixing, altering, or combining ingredients to fit the needs of an individual patient. Compounding is not the same as manufacturing. Large pharmaceutical companies, like Eli Lilly, make medications in strict, large-scale facilities that must follow FDA regulations for safety, purity, and consistency. By contrast, compounding is usually done in smaller pharmacies, often on a case-by-case basis. For example, a pharmacist may compound a medicine into a liquid form for a child who cannot swallow pills. Or they may prepare a medication without a certain dye if a patient is allergic.
The idea of compounding tirzepatide has grown in public attention because of a few key reasons. First, there have been periods when brand-name tirzepatide was in short supply. During shortages, patients and providers sometimes look for alternative ways to access the medication. Second, some people search for lower-cost versions, since brand-name medications are often expensive and not always covered by insurance. Compounding can sometimes appear to offer an affordable path. Third, not all patients can easily access brand-name tirzepatide through their health systems or local pharmacies, and compounding pharmacies may market themselves as filling that gap.
With all of this interest, patients and providers are asking many questions. Is it legal to compound tirzepatide in the United States? If it is legal, under what circumstances? How does compounded tirzepatide compare in terms of safety, quality, and effectiveness to FDA-approved versions like Mounjaro® or Zepbound®? Are there risks patients should know about? How much does it cost, and will insurance cover it?
The answers to these questions are not always simple, and in some cases, the answers are still evolving. The FDA has issued guidance about compounding GLP-1 medications, including tirzepatide, especially during drug shortages. At the same time, state boards of pharmacy regulate compounding practices in their own regions. Providers must also weigh the ethical and legal considerations when deciding whether to prescribe or recommend compounded versions of a brand-name drug.
For patients, the situation can be confusing. Many may hear about compounded tirzepatide from social media, news stories, or friends. Some may see online advertisements or receive emails from companies offering compounded versions. Without clear information, it can be hard to know which options are safe and which may put patients at risk.
For providers, the question is also complex. They must balance patient demand, drug availability, and regulatory requirements. Providers also need to consider their own professional responsibilities, since prescribing or recommending a compounded version of a drug involves different risks than prescribing an FDA-approved medication.
This article aims to provide a complete, clear, and balanced guide for both patients and providers about compounded tirzepatide. It will explain what compounding means, what the laws say, why some pharmacies may offer compounded tirzepatide, and what forms may be available. It will also look closely at safety, effectiveness, quality, and cost. Ethical and regulatory concerns will be discussed, and practical guidance will be given for both patients who are considering compounded tirzepatide and providers who may be asked about it.
The goal is not to give personal opinions or testimonials but to share evidence-based information in plain language. Because rules and availability may change over time, the information here reflects what is currently known but may continue to evolve. By the end of this guide, patients and providers should feel better prepared to understand the options, risks, and responsibilities related to compounded tirzepatide.
What Does It Mean to Compound Tirzepatide?
When people first hear the word “compounding,” it can sound complicated or even confusing. In reality, compounding is a long-standing part of pharmacy practice. It is the process of making a medicine in a way that is different from the product sold by a drug company. This can include changing the form, the strength, or the ingredients to meet a patient’s special medical needs.
What Is Drug Compounding?
Drug compounding is when a licensed pharmacist prepares a medication from raw ingredients, often called “active pharmaceutical ingredients” (APIs). Instead of using a pre-made pill or injection that is mass-produced by a pharmaceutical company, the pharmacist mixes and measures the drug in their pharmacy.
Compounding is not the same as manufacturing. A drug manufacturer produces large batches of medication in specialized facilities that follow strict Food and Drug Administration (FDA) approval standards. In contrast, compounding is typically done on a smaller scale, often one prescription at a time, for an individual patient.
Why Is Compounding Used?
Compounding exists to serve patients whose needs cannot be met by standard, mass-produced medications. Some of the main reasons include:
- Allergies or Sensitivities: A patient may need a medication without certain additives, such as dyes, preservatives, or fillers that are present in brand-name products.
- Different Dosage Form: A patient may have trouble swallowing pills and need the medicine in a liquid or other form.
- Custom Strengths: Some patients need doses that are not commercially available. Compounding allows the pharmacist to create the exact strength required.
- Drug Shortages: If a commercially available drug is in short supply, compounding may sometimes provide an alternative source.
When applied to tirzepatide, these reasons help explain why patients and providers may ask if it can be compounded.
What Is Tirzepatide?
Tirzepatide is a relatively new medication used for type 2 diabetes and, more recently, for chronic weight management. It works by activating two receptors in the body: the GLP-1 receptor and the GIP receptor. These actions help regulate blood sugar and control appetite.
The brand-name versions of tirzepatide available in the United States are:
- Mounjaro® – FDA-approved for type 2 diabetes.
- Zepbound® – FDA-approved for chronic weight management.
Both of these products come in pre-filled injection pens with specific doses.
How Does Compounding Apply to Tirzepatide?
When people ask, “Can tirzepatide be compounded?” they are usually referring to whether pharmacies can make their own versions of tirzepatide instead of using the brand-name injections.
Compounding tirzepatide would mean that a pharmacy takes the raw tirzepatide ingredient (the API) and prepares it into a medication for patients. This could include:
- Putting tirzepatide into vials for injection rather than using pre-filled pens.
- Creating custom doses that differ from the brand-name strengths.
- Preparing formulations that may exclude certain additives found in commercial products.
Compounding vs. Brand Manufacturing
It is important to understand the key differences:
- Standardization: Brand-name products like Mounjaro® and Zepbound® are made in large facilities with exact, consistent dosing and delivery systems. Compounded versions may vary in strength and stability, depending on the compounding process.
- Testing: FDA-approved medications go through years of research, clinical trials, and quality checks. Compounded drugs are not required to undergo the same level of testing.
- Regulation: Brand-name drugs are regulated by the FDA. Compounded medications are regulated at the state pharmacy level and by special sections of federal law.
Why the Question Matters
Tirzepatide is in very high demand because of its proven effects for both diabetes management and weight control. At times, shortages or high costs may drive patients and providers to look for alternatives. Compounding can seem like a solution, but it raises important questions about safety, legality, and effectiveness.
Is Compounding Tirzepatide Legal in the United States?
When people ask if tirzepatide can be compounded, one of the biggest concerns is whether it is legal. In the United States, drug compounding is allowed, but it is closely regulated. To understand the legality, it is important to first know how the Food and Drug Administration (FDA) oversees compounding, and what special rules apply to drugs like tirzepatide.
FDA Oversight of Compounded Drugs
The FDA is the agency that approves and monitors prescription drugs in the U.S. For a medication to be approved, the drug manufacturer must provide strong evidence from clinical trials showing that the drug is safe and effective. Tirzepatide, sold under brand names such as Mounjaro® and Zepbound®, is FDA-approved. This means the drug went through rigorous testing and the FDA confirmed that the medicine works and is safe when used as directed.
Compounded drugs are different. They are not FDA-approved. Instead, they are custom-made by pharmacists for individual patients. Because of this, compounded versions of a drug do not go through the same testing for safety, strength, or effectiveness as FDA-approved drugs. This difference is why the FDA has strict rules about when and how compounding can happen.
The Food, Drug, and Cosmetic Act
The main law that governs compounding is the Federal Food, Drug, and Cosmetic Act (FD&C Act). Within this law, there are two important sections that apply to pharmacies and outsourcing facilities:
- Section 503A – Traditional Pharmacy Compounding
- This section covers compounding by local pharmacies for specific patients.
- A pharmacist can only make the compounded drug if they have a valid prescription from a licensed healthcare provider.
- These compounded medicines are meant to meet special needs, such as changing a drug’s form (for example, making a liquid for someone who cannot swallow pills) or leaving out an ingredient due to an allergy.
- Pharmacies under 503A cannot make large batches for resale.
- This section covers compounding by local pharmacies for specific patients.
- Section 503B – Outsourcing Facilities
- This section allows larger compounding facilities, sometimes called outsourcing pharmacies, to prepare compounded drugs in bulk.
- These facilities are subject to more oversight, similar to drug manufacturers, including inspections and quality standards.
- 503B compounding is often used to address drug shortages.
- This section allows larger compounding facilities, sometimes called outsourcing pharmacies, to prepare compounded drugs in bulk.
Both 503A pharmacies and 503B outsourcing facilities must follow state and federal rules. They must also use ingredients that meet specific standards, such as those in the United States Pharmacopeia (USP).
FDA Guidance for Compounding Tirzepatide
The FDA has given special warnings about compounding GLP-1 and GIP receptor agonists, the class of drugs that tirzepatide belongs to. The FDA has stated that:
- Compounding is only allowed when there is a shortage of the FDA-approved product.
- Once the shortage is resolved, pharmacies should not continue making compounded versions.
- Compounded tirzepatide must be made with the correct active pharmaceutical ingredient (API) that meets quality standards.
The FDA has also warned against pharmacies or wellness clinics that sell tirzepatide in forms or strengths that are not tested or approved. For example, some compounded versions have been marketed as “oral tirzepatide” or in non-standard doses. These products may not be legal and could pose safety risks.
Why Legal Questions Arise Around Tirzepatide
Legal questions about compounding tirzepatide are common for several reasons:
- Drug Shortages: At times, patients have had trouble accessing Mounjaro® or Zepbound®. During shortages, compounding may be temporarily allowed.
- State Differences: Each state has its own board of pharmacy, which sets rules on compounding. What is legal in one state may not be in another.
- Marketing Practices: Some compounding pharmacies advertise tirzepatide for weight loss in ways that may not align with FDA rules. This can confuse patients and providers about what is legal.
- Safety Concerns: Because compounded drugs are not FDA-approved, the agency regularly reminds the public that compounded tirzepatide is not the same as the brand-name drug.
Legally, tirzepatide can be compounded in the U.S., but only in narrow situations, mainly during shortages or when a patient has a specific medical need that FDA-approved drugs cannot meet. Compounded tirzepatide is not the same as the FDA-approved versions, and it does not go through the same testing for safety and effectiveness. Both patients and providers should carefully review the rules and ensure any compounded medication comes from a legitimate source.

Why Are Some Pharmacies Offering Compounded Tirzepatide?
When patients search for tirzepatide, they often come across advertisements or websites from pharmacies that sell “compounded tirzepatide.” This can be confusing because tirzepatide already exists as an FDA-approved medicine under the brand names Mounjaro® (for type 2 diabetes) and Zepbound® (for obesity and weight management). So why would pharmacies need to make a compounded version of the same drug? The answer has several parts, including drug shortages, high demand, differences in state rules, and patient access.
Medication Shortages
One of the biggest reasons pharmacies began offering compounded tirzepatide is due to shortages of the brand-name products. In the last few years, demand for medicines like Mounjaro® and Zepbound® has been extremely high. More and more people are asking for these medicines to help with diabetes or weight loss.
When demand is greater than supply, manufacturers sometimes cannot keep up. This has happened with other injectable medicines too, such as insulin or semaglutide. If a medicine is officially listed by the FDA as being “in shortage,” certain compounding pharmacies are legally allowed to prepare their own versions to help fill the gap for patients.
Shortages can last for months or even longer, and patients may not be able to find their medicine at local drugstores. Compounding pharmacies sometimes step in to give patients another option when the approved drug is not available.
Patient Demand for Weight Management
Another reason is patient demand. Tirzepatide works by acting on both GLP-1 and GIP receptors, which can lower blood sugar and reduce appetite. Because of these effects, many people who do not have diabetes are interested in the medication for weight loss.
Zepbound® was approved in late 2023 for obesity, but even before that approval, doctors and patients were asking about using tirzepatide off-label for weight management. With so many people searching for it, interest quickly grew beyond the original supply that was meant for people with diabetes.
When people cannot find the brand-name version, some turn to compounding pharmacies to get what looks like a similar product. Compounding pharmacies may advertise that they can prepare tirzepatide in different strengths or sell it at lower cost.
State-Level Pharmacy Rules
In the United States, compounding pharmacies are regulated both by state pharmacy boards and, in some cases, by federal law. Each state has its own rules about what pharmacies can and cannot compound.
Some states allow pharmacies to prepare compounded versions of medicines if the FDA lists them as being in shortage. Others may allow compounding if a doctor writes a prescription stating the patient cannot get the brand product.
Because the rules are not the same across the country, patients may see compounded tirzepatide offered in some states but not in others. This patchwork of rules has created confusion. Some patients assume that because one pharmacy is offering it online, it must be legal everywhere—but that is not always true.
Access and Cost Issues
Access also plays a big role. Even when the brand medicines are available, not every patient can afford them. Mounjaro® and Zepbound® can cost hundreds to more than a thousand dollars per month without insurance. Insurance companies may not cover these medicines, especially for weight loss, since many policies only cover them for type 2 diabetes.
Because of this, some patients look to compounding pharmacies as a cheaper option. These pharmacies may market compounded tirzepatide at lower prices, claiming it gives patients access to a similar medicine for less money.
However, lower cost does not always mean equal quality. FDA-approved products go through strict testing for safety, effectiveness, and sterility. Compounded medicines do not go through the same process, which is why the FDA warns patients and providers to be cautious.
Marketing and Awareness
Finally, there is the issue of marketing. Some compounding pharmacies actively promote tirzepatide on social media and online platforms. They highlight its role in weight loss and use terms like “compounded GLP-1” or “customized injections.”
This kind of advertising can make compounded tirzepatide sound like a normal, safe, and widely accepted option. But the truth is that compounded versions do not have the same scientific evidence or FDA approval behind them. Marketing can sometimes make the difference seem smaller than it really is.
What Forms of Compounded Tirzepatide Are Available?
When patients and providers hear about compounded tirzepatide, one of the first questions is what form the medication comes in. Since compounded drugs are not made by large pharmaceutical companies, they can be prepared in different ways by compounding pharmacies. This section explains the common forms of compounded tirzepatide, how they compare to FDA-approved brand-name products, and what safety concerns to keep in mind.
FDA-Approved Forms vs. Compounded Forms
The FDA-approved version of tirzepatide is sold under brand names such as Mounjaro® (for type 2 diabetes) and Zepbound® (for weight management). These brand products are only available as prefilled injection pens. Each pen is carefully tested for dose accuracy, sterility, and stability before being sold.
Compounded tirzepatide, however, can take other forms. Compounding pharmacies may prepare tirzepatide as:
- Multi-dose vials for injection
- Prefilled syringes
- Oral formulations (capsules, sublingual drops, or troches/lozenges)
These forms are not standardized or reviewed by the FDA. Their preparation and quality may vary depending on the pharmacy.
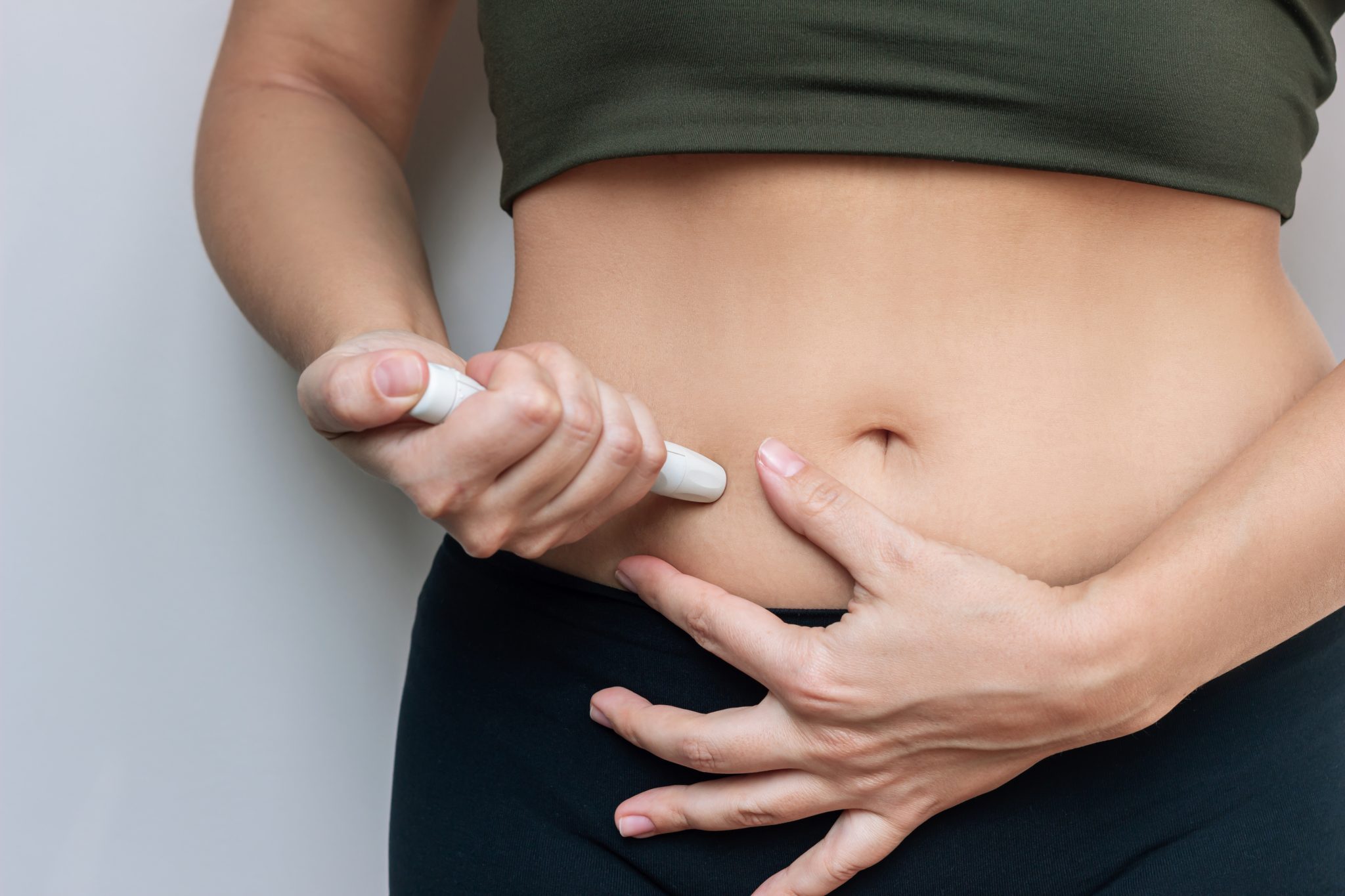
Injectable Compounded Tirzepatide
Most compounded tirzepatide is provided as injectable medicine. This is the form that most closely matches the FDA-approved version. Compounding pharmacies usually prepare tirzepatide as:
- Multi-dose vials: A small glass vial containing sterile liquid tirzepatide. Patients use an insulin-type syringe to measure and inject their dose.
- Prefilled syringes: Syringes already filled with the medication at a set dose, ready to use.
Differences from brand products:
- Dosing flexibility: Some pharmacies may offer compounded tirzepatide in strengths that are not available with Mounjaro® or Zepbound®. For example, they may provide custom concentrations or allow for smaller dose adjustments.
- Risk of dosing errors: Unlike the prefilled pens from brand companies, which “click” to deliver an exact amount, compounded vials require patients to measure their own dose. This increases the risk of incorrect dosing if not carefully done.
- Sterility and contamination risk: Multi-use vials must be handled carefully to avoid contamination each time a needle enters the vial. Pharmacies must follow strict sterile compounding rules, but not all facilities meet the same quality standards.
Oral Forms of Compounded Tirzepatide
Some compounding pharmacies market oral versions of tirzepatide, such as:
- Capsules
- Sublingual drops (placed under the tongue)
- Troches (small lozenges that dissolve in the mouth)
These oral forms are not FDA-approved. The effectiveness of tirzepatide by mouth is not well studied, since tirzepatide is a peptide drug that may break down in the digestive system.
Concerns with oral compounded tirzepatide:
- Absorption: It is unclear how much of the drug is absorbed into the bloodstream when taken by mouth. The FDA-approved version is only injectable because injections ensure the medicine gets into the body without being broken down by stomach acids and enzymes.
- Effectiveness: Because absorption is uncertain, patients may not get the same blood levels of the drug as they would with an injection. This means the effect on blood sugar or weight could be weaker or unpredictable.
- Stability: Oral forms may not remain stable or effective for long periods, depending on how they are stored.
Strength, Dosage, and Customization
One reason patients and providers turn to compounding pharmacies is the ability to customize doses. With brand-name products, Mounjaro® and Zepbound® pens come in fixed strengths. Compounded tirzepatide may allow for:
- Smaller starting doses for patients who are sensitive to side effects.
- Intermediate doses between the standard steps used in brand products.
- Different vial sizes for cost or convenience.
While flexibility can be helpful, it also introduces risk. Without the same strict quality checks as brand-name drugs, there is more room for variability in potency and dosing accuracy.
Quality and Sterility Considerations
All compounded injectable tirzepatide should be prepared under sterile conditions in line with United States Pharmacopeia (USP) standards. Pharmacies that do not follow these standards may produce medication with:
- Contamination by bacteria or particles, which can be harmful if injected.
- Incorrect concentration of drug, leading to too little or too much medicine per dose.
- Poor stability, meaning the drug loses strength over time.
Patients should be aware that the FDA has issued warnings about compounded GLP-1 and GIP receptor agonists. These warnings stress that compounded forms may carry safety risks, especially when purchased from unlicensed or online-only sources.
Compounded tirzepatide can come in different forms, most often as injectable vials or syringes, but sometimes as oral capsules or lozenges. These compounded forms differ from the FDA-approved prefilled injection pens of Mounjaro® and Zepbound®. While compounding allows for flexibility in dosing, it also raises concerns about sterility, stability, and effectiveness—especially for oral versions that have not been tested in large clinical trials.
For safety, patients and providers should discuss carefully whether a compounded form is appropriate and verify that the pharmacy follows strict sterile compounding standards.
How Does the Safety of Compounded Tirzepatide Compare to FDA-Approved Products?
When patients and providers consider compounded tirzepatide, one of the biggest questions is whether it is as safe as the brand-name medications, such as Mounjaro® or Zepbound®. To answer this, it is important to understand how the U.S. Food and Drug Administration (FDA) approves medicines, how compounding works, and where risks may appear.
FDA-Approved Safety Standards
FDA-approved medications go through years of testing before reaching the public. This includes:
- Preclinical studies in labs and animals to learn about safety and how the drug works.
- Large clinical trials with thousands of people to prove the medicine is safe and effective for specific health conditions.
- Strict manufacturing standards under “Good Manufacturing Practices” (GMP). These rules require exact dosing, strong quality controls, and repeated safety checks at every step of production.
For brand-name tirzepatide (Mounjaro® for type 2 diabetes and Zepbound® for weight management), these processes mean every dose is consistent, sterile, and meets the same high-quality standard. Patients and providers can trust that each injection is the same strength and free of harmful contamination.
How Compounded Medications Are Different
Compounded tirzepatide does not go through the same process. Compounding pharmacies prepare medicines for individual patients, often when an FDA-approved drug is unavailable or unsuitable. Examples include making a liquid form for someone who cannot swallow pills or preparing a drug without an ingredient that causes allergies.
However, there are major differences:
- No FDA approval: Compounded drugs are not reviewed or approved by the FDA.
- No large safety trials: There are no clinical studies proving compounded tirzepatide is safe or effective.
- Variable quality: Each pharmacy may use different sources of ingredients, different preparation methods, and different storage systems.
This means the safety of compounded tirzepatide can vary from one pharmacy to another.
Potential Safety Risks of Compounded Tirzepatide
- Contamination
Tirzepatide must be prepared in sterile conditions, because it is injected under the skin. If a pharmacy does not follow strict sterile techniques, bacteria or other contaminants can get into the medication. This can lead to serious infections.
- Incorrect Dosing
FDA-approved tirzepatide pens are made to deliver precise doses. With compounded versions, there may be errors in measuring the amount of active drug. Too little means the medicine may not work. Too much may cause stronger side effects, such as nausea, vomiting, or dangerously low blood sugar when combined with other diabetes medicines.
- Potency Variability
The strength of compounded medications can vary. In some cases, testing has shown compounded drugs containing either more or less active ingredient than listed. This lack of consistency can make it harder for providers to adjust doses safely.
- Unapproved Ingredients or Additives
Some compounded versions may include different preservatives, stabilizers, or inactive ingredients. These can affect how the drug works or cause allergic reactions.
FDA Warnings and Safety Alerts
The FDA has released warnings about compounded versions of GLP-1 receptor agonists, including tirzepatide and semaglutide. These warnings highlight concerns that:
- Some pharmacies are selling compounded tirzepatide without meeting proper compounding rules.
- Some products have been found with incorrect ingredients.
- In some cases, patients have reported adverse events linked to compounded injections.
Because of these risks, the FDA advises patients to use FDA-approved products whenever possible and to be cautious about compounded versions.
Why Safety Is Hard to Guarantee
The key issue is standardization. An FDA-approved drug like Mounjaro® has identical quality no matter where or when it is purchased. In contrast, compounded tirzepatide can differ widely depending on:
- Which raw materials are used (and where they come from).
- How the pharmacy prepares the injection.
- Whether the pharmacy follows strict sterile compounding rules (USP <797> standards).
- How the medication is stored and transported.
This variability means one batch of compounded tirzepatide may be safe, but another may not.
What Patients and Providers Should Keep in Mind
- Compounded tirzepatide is not the same as Mounjaro® or Zepbound®. Even if the label says “tirzepatide,” the safety and quality are not guaranteed to match the brand-name drugs.
- Risks can be serious. Infections, allergic reactions, or incorrect dosing can all cause harm.
- Compounded drugs should only be used when necessary. For example, during shortages of FDA-approved products or if a patient has no access to approved versions.
- Close medical monitoring is essential. Providers should carefully track blood sugar, weight changes, side effects, and overall health when a patient is on compounded tirzepatide.
Compounded tirzepatide may look similar to Mounjaro® or Zepbound®, but the safety profile is very different. Without FDA oversight, compounded versions carry higher risks, including contamination, incorrect dosing, and variable strength. For patients and providers, it is important to weigh these risks carefully and to use compounded tirzepatide only when FDA-approved products are not available. Safety should always come first, and treatment should always be guided by a licensed healthcare professional.

Are There Differences in Effectiveness Between Compounded and Brand-Name Tirzepatide?
When patients and providers think about compounded tirzepatide, one of the most important questions is whether it works the same way as the FDA-approved brand-name products, such as Mounjaro® or Zepbound®. Understanding this requires looking at what we know about brand-name medications, and then comparing that to what is known — and what is not known — about compounded versions.
Effectiveness of FDA-Approved Tirzepatide
Tirzepatide is a medication that acts on both the GLP-1 receptor and the GIP receptor in the body. By working on these pathways, it helps control blood sugar levels and reduces appetite, which supports weight loss.
The FDA approval of Mounjaro® and Zepbound® was based on large clinical trials. These studies involved thousands of patients with type 2 diabetes and people living with obesity. The results showed:
- Significant improvements in blood sugar control (measured by A1C levels).
- Large and consistent reductions in body weight.
- A generally safe profile, with side effects that were mostly gastrointestinal, such as nausea or diarrhea.
Because these results came from well-controlled, carefully monitored trials, we can be confident about how effective brand-name tirzepatide is. Every vial or pen of Mounjaro® or Zepbound® has the same strength, purity, and safety profile.
What We Know (and Don’t Know) About Compounded Tirzepatide
Compounded medications are not studied in the same way. Compounded tirzepatide has not been tested in large clinical trials. Instead, these versions are prepared in smaller batches by compounding pharmacies, often in response to shortages of the brand-name drug.
This means:
- No standardized clinical data: We cannot point to large studies proving compounded tirzepatide has the same effect on weight loss or blood sugar control.
- Variability between pharmacies: The strength, quality, and consistency of compounded formulations may differ from one pharmacy to another.
- Unknown long-term outcomes: We don’t have reliable data on how well compounded tirzepatide works over months or years compared to FDA-approved products.
Possible Factors That Influence Effectiveness
Even if compounded tirzepatide contains the same active ingredient, several factors can change how effective it is in the body:
- Formulation Differences
- Brand-name tirzepatide comes in prefilled injection pens with carefully designed delivery systems.
- Compounded versions may be provided in vials with syringes, or even in oral forms, which could change how the drug is absorbed or used by the body.
- Brand-name tirzepatide comes in prefilled injection pens with carefully designed delivery systems.
- Dosing Accuracy
- Mounjaro® and Zepbound® pens deliver exact, pre-measured doses.
- With compounded vials, patients or providers may need to measure doses manually. Small mistakes in measurement can affect effectiveness and safety.
- Mounjaro® and Zepbound® pens deliver exact, pre-measured doses.
- Stability and Storage
- Brand-name products undergo stability testing to ensure the medication stays effective up to the expiration date when stored correctly.
- Compounded versions may not have the same level of stability testing, so potency could be reduced if storage conditions are not ideal.
- Brand-name products undergo stability testing to ensure the medication stays effective up to the expiration date when stored correctly.
- Sterility and Purity
- Contamination or impurities in compounded products can reduce effectiveness and increase risks.
- FDA-approved products are manufactured under strict standards to prevent these problems.
- Contamination or impurities in compounded products can reduce effectiveness and increase risks.
Reported Patient Experiences (With Caution)
Some patients report that compounded tirzepatide helps them achieve blood sugar control or weight loss similar to brand-name medications. However, these reports are individual stories, not large studies. Without controlled trials, we cannot be certain if the results are consistent across all patients.
It is also important to remember that the FDA has released safety alerts warning that some compounded GLP-1 medications may contain the wrong ingredients or incorrect strengths. Such variations can directly affect how well the medication works.
For these reasons, providers often emphasize that while compounded tirzepatide may sometimes be used during shortages, its effectiveness compared to FDA-approved products cannot be guaranteed. Patients considering compounded versions should discuss the risks, benefits, and monitoring plan with their healthcare provider before starting treatment.
How Can Patients and Providers Verify the Quality of Compounded Tirzepatide?
When a medication is made by a large pharmaceutical company, it must go through strict FDA approval before reaching patients. The company must prove the medicine is safe, effective, and consistent in strength and purity. With compounded medications, this process is different. Compounded tirzepatide is prepared by a licensed pharmacy, usually when the FDA-approved versions like Mounjaro® or Zepbound® are not available or cannot meet a patient’s specific needs. Because compounded drugs are not FDA-approved, the responsibility to ensure quality and safety often shifts to patients and providers.
Below are important ways patients and healthcare professionals can work together to verify the quality of compounded tirzepatide.
Accreditation Systems and Why They Matter
One of the best ways to judge the reliability of a compounding pharmacy is to look for accreditation. A key program in the United States is the Pharmacy Compounding Accreditation Board (PCAB).
- What PCAB accreditation means:
Pharmacies with this certification must meet extra standards for quality, cleanliness, and safety. These standards go beyond the minimum state pharmacy rules. Inspectors check that the pharmacy follows correct procedures for sterile medications, tests for potency, and ensures consistency from batch to batch. - Why it matters for tirzepatide:
Since tirzepatide is an injectable medication, sterility is critical. Any contamination can cause infections or serious harm. Choosing a PCAB-accredited pharmacy reduces the risks, though it does not guarantee the medication is identical to the brand-name version.
Providers should check whether a pharmacy has PCAB accreditation and share that information with their patients before prescribing or recommending compounded tirzepatide.
Questions Patients Should Ask a Compounding Pharmacy
Patients often feel unsure about what to ask when calling or visiting a compounding pharmacy. Having a list of questions can make the process less intimidating and give confidence in the medication’s safety.
Some useful questions include:
- “Is your pharmacy PCAB accredited?”
Accreditation does not replace FDA approval, but it shows the pharmacy meets higher quality standards. - “Do you perform potency and sterility testing on your compounded medications?”
Reliable pharmacies should be able to provide documentation showing the medication has been tested. - “Can you provide a certificate of analysis (COA) for my medication?”
A COA is a report that confirms the strength and purity of the drug batch. It is a sign the pharmacy is testing and monitoring quality. - “Where do you source your raw ingredients for tirzepatide?”
Ingredients should come from suppliers registered with the FDA or other regulated agencies. - “How should this medication be stored, and what is its beyond-use date?”
Compounded medications often have shorter shelf lives than brand-name drugs. Patients should know how long the product is stable and how to store it safely.
The Role of Providers in Oversight
Healthcare providers play a critical role in protecting patients when compounded tirzepatide is considered. Unlike patients, providers can evaluate scientific details and make medical decisions based on a risk-benefit analysis.
- Evaluating the need: A provider should first ask whether compounded tirzepatide is necessary. If the FDA-approved version is available and affordable, it is usually the safest option.
- Choosing pharmacies carefully: Providers should only work with compounding pharmacies they trust. They can research the pharmacy’s history, look for disciplinary actions, and verify licensure.
- Monitoring therapy: Once a patient begins compounded tirzepatide, providers should closely monitor blood sugar, weight, and any side effects. They should also keep records of the product used, the batch number, and the compounding pharmacy, in case any safety concerns arise later.
Warning Signs of Poor-Quality Compounded Medications
Patients and providers should stay alert for red flags that may signal poor compounding practices. These can include:
- Medication that looks cloudy, discolored, or has floating particles.
- Packaging that appears unprofessional, lacks clear labeling, or does not include instructions.
- A pharmacy unwilling to answer questions or provide test results.
- Prices that seem much lower than competitors, which may suggest cutting corners on quality.
If any of these warning signs appear, patients should stop using the product and contact their provider right away.
Importance of Ongoing Communication
Even when a trusted pharmacy is used, ongoing communication between patients and providers is essential. Patients should report side effects, changes in how the medication seems to work, or any doubts about the medication’s appearance. Providers can then decide whether adjustments, lab tests, or switching pharmacies are needed.
This teamwork helps to reduce risks and protect patient safety while using a compounded product.
By being proactive, patients and providers can work together to verify the quality of compounded tirzepatide and reduce the risks that come with using a non-FDA-approved product.
Cost and Insurance Coverage: Does Compounded Tirzepatide Save Money?
The cost of medication is one of the main reasons patients and providers consider compounded tirzepatide. Many people ask whether compounded versions are cheaper than the brand-name products and if insurance will help cover the cost. In this section, we will look at how much compounded tirzepatide usually costs, how it compares to FDA-approved products like Mounjaro® and Zepbound®, what patients can expect with insurance coverage, and the risks of focusing only on cost.
Typical Pricing for Compounded Tirzepatide
Pharmacies that compound tirzepatide often advertise it as being less expensive than brand-name injections. In many cases, a compounded version may be hundreds of dollars less per month than Mounjaro® or Zepbound®, which can cost over $1,000 per month without insurance. Some compounding pharmacies list prices around $200–$500 per month, though this can vary depending on the dose, the pharmacy’s location, and whether the medication is prepared as a vial or pre-filled syringe.
However, patients should know that lower price does not always mean the same value. Brand-name medications are FDA-approved, meaning every vial or pen must meet strict standards for purity, potency, and sterility. Compounded medications are not tested in the same way. That means the cheaper cost may come with trade-offs in safety, consistency, or effectiveness.
Cost Differences Between Branded and Compounded Versions
FDA-approved tirzepatide is sold by Eli Lilly under two brand names:
- Mounjaro® – approved for type 2 diabetes.
- Zepbound® – approved for chronic weight management.
Both are delivered as single-use injection pens and are priced at similar levels. Without insurance, monthly costs are high, which has pushed some patients to seek compounded versions.
Compounded tirzepatide may be offered as a multi-dose vial, where patients use their own syringes, or in other forms not sold by the manufacturer. These changes reduce costs for pharmacies but also introduce new risks, such as contamination when withdrawing multiple doses from the same vial.
While a compounded vial may cost less upfront, patients may need to consider additional costs for supplies, safe storage, and medical visits to monitor for side effects.
Insurance Coverage Challenges
One of the most important points for patients to understand is that insurance rarely covers compounded tirzepatide. Health plans usually only cover FDA-approved products that have been tested for safety and effectiveness. Because compounded tirzepatide is not FDA-approved, most insurers will not pay for it.
In contrast, some insurance plans may cover part of the cost for Mounjaro® or Zepbound® if the patient meets certain criteria. For example, coverage may be approved for type 2 diabetes when other medications have not worked, or for obesity if the patient has a qualifying body mass index (BMI) with related health risks. Even then, many patients face high copayments or must meet strict requirements before approval.
This difference means that although compounded tirzepatide looks less expensive at first, the true out-of-pocket cost could be similar or even higher if brand-name medication is covered by insurance but compounded medication is not.
Out-of-Pocket Considerations for Patients
Patients who choose compounded tirzepatide usually must pay the entire cost themselves. For some, paying $300–$500 per month may still be more manageable than paying the full cash price of Mounjaro® or Zepbound®. But these costs can add up quickly, especially since treatment for diabetes or weight management is often long-term.
Other hidden costs include:
- Doctor visits for follow-up and monitoring.
- Lab tests to check blood sugar, liver function, or other health markers.
- Supplies such as syringes or alcohol swabs if the medication comes in a vial.
It is important for patients to look at the whole picture of costs, not just the pharmacy price.
Weighing Cost Against Safety
While cost savings are important, patients and providers should not make decisions based only on price. FDA has issued warnings about compounded tirzepatide products that may contain incorrect doses, harmful impurities, or even ingredients that are not tirzepatide at all. These problems may put patients at risk of dangerous side effects, poor blood sugar control, or wasted money if the drug is not effective.
Some patients may spend less each month on compounded tirzepatide but face higher costs later if unsafe medication leads to complications. Hospital visits, extra testing, or switching therapies can all increase expenses far beyond the savings.

What Are the Ethical and Regulatory Concerns Surrounding Compounded Tirzepatide?
Compounded medications are not new. Pharmacists have been compounding drugs for decades to meet special needs, such as when a patient is allergic to an ingredient in a brand-name drug or requires a unique strength that is not sold by the manufacturer. However, when it comes to powerful medicines like tirzepatide, the questions of ethics, safety, and regulation become more complex. Patients and providers need to understand these concerns before making decisions about compounded tirzepatide.
Balancing Access and Safety
One of the biggest ethical questions is how to balance patient access to treatment with patient safety. Many people want tirzepatide because of its proven benefits for diabetes and weight management. However, branded products like Mounjaro® and Zepbound® have been in short supply at times, leaving patients without access.
When a drug is in shortage, the FDA sometimes allows compounding pharmacies to step in and prepare versions of the medication. This gives patients access they would not otherwise have. But compounding does not go through the same strict approval process as the original drug. That means patients may get access more quickly but may also face higher risks.
Providers have to weigh these trade-offs. Is it better for a patient to go without treatment or to use a compounded form that may not meet the same quality standards? This is the heart of the ethical debate.
Risks of Misuse and Counterfeiting
Another concern is the potential for misuse. Because compounded tirzepatide is often sold outside the normal supply chain, there is a higher chance of mistakes or even fraud.
- Dosing errors: Unlike FDA-approved tirzepatide, compounded versions may vary in strength. A small error in preparation can lead to underdosing (which makes the drug less effective) or overdosing (which can increase side effects and complications).
- Counterfeit products: Some online sellers may claim to offer compounded tirzepatide but instead sell fake products. These may contain no active drug, the wrong drug, or harmful ingredients. Patients who buy from unverified sources face serious risks.
For these reasons, the FDA has issued warnings about purchasing tirzepatide and similar drugs from unregulated online pharmacies or social media marketplaces.
Gaps in Regulation
FDA-approved medications like Mounjaro® and Zepbound® are made under strict conditions that ensure every vial or pen contains the exact same dose and is free of harmful contamination. Compounding pharmacies do not have to meet those same large-scale manufacturing standards. Instead, they follow state pharmacy board rules, which can vary.
There are two main types of compounding pharmacies:
- 503A pharmacies – These are traditional compounding pharmacies that prepare medications for individual patients based on a prescription. Their oversight is mainly at the state level.
- 503B outsourcing facilities – These are larger operations that can produce compounded medications in bulk. They are subject to more FDA oversight, but the standards are still not as strict as those for brand-name manufacturers.
Because of these differences, there may be inconsistencies in quality, sterility, or potency between pharmacies. Patients and providers cannot assume that compounded tirzepatide from one source will be the same as from another.
Ethical Concerns for Providers
Healthcare providers also face ethical questions when prescribing or recommending compounded tirzepatide. They must consider:
- Informed consent: Patients should be told clearly that compounded tirzepatide is not FDA-approved and may carry different risks.
- Clinical responsibility: Providers need to monitor patients closely for both effectiveness and side effects. This may require more frequent visits and lab tests.
- Professional boundaries: Some providers may feel pressure from patients to prescribe compounded tirzepatide because it can be easier to get or cheaper. Providers must balance patient wishes with medical safety and regulatory rules.
Ongoing Legal and Professional Debates
The debate about compounded tirzepatide is still unfolding. Regulators, manufacturers, pharmacists, and healthcare providers all have different perspectives:
- Regulators want to protect public health by ensuring that medications are safe and effective.
- Pharmacists want to help fill gaps in access when brand-name products are unavailable.
- Providers want to give their patients treatment options without putting them at unnecessary risk.
- Manufacturers argue that compounded products may undermine safety and the value of their FDA-approved drugs.
These groups often disagree on where the line should be drawn. The FDA has already issued statements that compounding tirzepatide is not allowed when there is no shortage. But when shortages do occur, the rules become less clear, leaving room for debate.
Compounded tirzepatide raises important questions that go beyond simple access to medication. The issues involve safety risks, regulatory gaps, potential misuse, and the ethical responsibility of healthcare providers. While compounded drugs may help patients during shortages, they come with trade-offs that patients and providers must carefully consider.
For now, the safest path is for patients to have open discussions with licensed providers and only obtain medications from trusted, state-licensed, or FDA-registered compounding pharmacies. As rules continue to evolve, staying informed will be the key to making safe and ethical choices.
Guidance for Patients Considering Compounded Tirzepatide
If you are thinking about using compounded tirzepatide, it is important to take time to understand what it means, what risks it may carry, and what questions you should ask before starting. Compounded medications can sometimes help patients who cannot get brand-name drugs. However, they are not the same as the products that the U.S. Food and Drug Administration (FDA) has approved. This section gives step-by-step guidance for patients who want to make safe and informed choices.
Step 1: Talk to a Licensed Healthcare Provider First
The first step is to have an honest conversation with your doctor, nurse practitioner, or other licensed healthcare provider. Tell them why you are interested in compounded tirzepatide. Common reasons include difficulty accessing the brand-name medications Mounjaro® (approved for type 2 diabetes) or Zepbound® (approved for weight management), or concerns about cost.
Your provider can:
- Review your medical history.
- Check if tirzepatide is safe for you.
- Explain the difference between FDA-approved and compounded forms.
- Discuss other options if compounding does not seem safe or necessary.
This step is important because some patients may not be good candidates for tirzepatide at all, even if it is compounded. For example, people with certain endocrine conditions, pancreas problems, or a history of severe gastrointestinal disease may face higher risks.
Step 2: Ask Key Questions About the Compounded Medication
If your provider thinks compounded tirzepatide might be reasonable, the next step is to learn more about the exact product you would receive. Patients should not be afraid to ask questions. Some important ones include:
- Where is the medication made? Make sure the pharmacy is licensed in your state.
- Is the pharmacy accredited? Accreditation by organizations like the Pharmacy Compounding Accreditation Board (PCAB) shows a commitment to safety and quality.
- How is the drug prepared? Ask if it is sterile, since injections require the highest safety standards.
- How is the strength measured? Compounded medications must be carefully dosed, and mistakes can lead to either underdosing or overdosing.
- What proof of testing is provided? Quality testing for potency and sterility is a good sign of reliability.
Patients should also confirm that the label on the compounded tirzepatide includes clear instructions, expiration dates, and storage directions.
Step 3: Understand the Risks of Compounded Tirzepatide
Unlike FDA-approved Mounjaro® and Zepbound®, compounded tirzepatide does not go through the same strict clinical trials or large-scale safety checks. This means there are some risks:
- Variability in potency: The actual strength may be higher or lower than expected.
- Contamination: If the product is not made under sterile conditions, infections could occur.
- Limited oversight: Compounded drugs are monitored by state pharmacy boards but not held to the same federal approval standards.
Understanding these risks helps patients avoid assuming that compounded drugs are “just the same” as the brand-name version.
Step 4: Be Clear on Cost and Coverage
For many patients, cost is one reason to consider compounded tirzepatide. Compounded medications are often cheaper than branded products. However, most insurance plans do not cover them. This means you may need to pay the full amount out of pocket.
Before starting, ask the pharmacy for a written cost estimate. Compare that with the cost of the brand-name product, especially if you can access patient assistance programs from the drug manufacturer.
Step 5: Follow Storage and Use Instructions Exactly
If you and your provider decide to move forward, make sure you know how to store and use the medication safely. Some compounded tirzepatide may need refrigeration. Others may be stable at room temperature for only a short period. Improper storage can affect safety and effectiveness.
When it comes to injecting tirzepatide, your provider or pharmacist should show you:
- How to prepare the dose.
- Where and how to inject safely.
- How to rotate injection sites to avoid irritation.
Never change the dose on your own. Only adjust it if your provider tells you to.
Step 6: Stay in Regular Contact With Your Provider
Once you start compounded tirzepatide, you should have close follow-up. This allows your provider to:
- Monitor blood sugar or weight changes.
- Watch for side effects such as nausea, vomiting, or abdominal pain.
- Adjust the dose if needed.
- Reassess whether compounded medication is still appropriate.
If you experience severe side effects, such as signs of pancreatitis (sudden severe stomach pain), stop taking the medication and call your provider right away.
Step 7: Stay Informed About FDA Updates
Rules about compounded tirzepatide can change. The FDA may update its guidance if drug shortages improve or if new safety concerns are discovered. Patients should stay informed and ask their provider if there have been any new warnings.
Compounded tirzepatide may help some patients when brand-name products are hard to find or too expensive. But it also carries extra risks that patients should not overlook. By working closely with a licensed provider, asking the right questions, checking pharmacy credentials, and carefully following instructions, you can make a safer and more informed decision about whether compounded tirzepatide is right for you.
Guidance for Providers Managing Patients on Compounded Tirzepatide
Managing patients who are considering or already using compounded tirzepatide requires careful thought and extra steps for safety. Providers play an important role in balancing patient needs, regulatory rules, and clinical best practices. This section explains how healthcare providers can approach the use of compounded tirzepatide in a responsible and informed way.
Risk–Benefit Assessment Before Prescribing or Recommending
The first step is to carefully weigh the risks and benefits. FDA-approved tirzepatide products, such as Mounjaro® and Zepbound®, have gone through strict testing for safety, quality, and effectiveness. Compounded versions, by contrast, do not have the same level of oversight.
When considering compounded tirzepatide, providers should ask:
- Is there a shortage of the brand-name drug? FDA rules generally allow compounding only if the approved drug is unavailable or cannot meet patient needs.
- Does the patient have a specific medical reason for compounding? For example, a patient who needs a different dosage form or strength may qualify.
- Are the risks acceptable compared with the possible benefits? Patients should understand that compounded drugs may carry higher risks of dosing errors or contamination.
Documenting this risk–benefit assessment is important for both patient safety and legal protection.
Best Practices for Documentation and Informed Consent
Clear documentation protects both patients and providers. Every decision related to compounded tirzepatide should be written in the patient’s medical record. This includes:
- Why compounded tirzepatide is being considered.
- Notes about discussions with the patient on risks and alternatives.
- The name and details of the compounding pharmacy used.
Informed consent is a key part of this process. Patients should be given a simple, written explanation that includes:
- The difference between FDA-approved and compounded drugs.
- The fact that compounded tirzepatide has not been tested in the same way as approved versions.
- Possible safety risks, including changes in potency, sterility issues, and lack of insurance coverage.
The patient should sign the consent form to show they understand and agree. This step builds trust and reduces misunderstandings later.
Clinical Monitoring Strategies for Safety and Effectiveness
Once a patient begins compounded tirzepatide, providers need to set up a clear monitoring plan. The goals are to ensure the medication is working as intended and to detect any problems early.
Suggested monitoring steps include:
- Baseline assessment – Record weight, blood sugar, blood pressure, kidney function, and other health markers before starting.
- Early follow-up – Schedule an appointment or check-in within the first 4–6 weeks. Ask about side effects such as nausea, vomiting, or injection site reactions.
- Regular monitoring – Check A1C, fasting glucose, and weight every few months, similar to patients using brand-name tirzepatide.
- Adverse event reporting – Encourage patients to report any unusual symptoms right away. Providers should also report serious issues to the FDA MedWatch system, especially if they may be linked to compounding quality.
If the compounded medication seems ineffective or unsafe, providers should re-evaluate the treatment plan and consider switching back to an FDA-approved version when available.
Choosing and Working With a Compounding Pharmacy
Not all compounding pharmacies are equal. Providers should try to partner only with pharmacies that:
- Are licensed in the provider’s state.
- Follow United States Pharmacopeia (USP) standards for sterile compounding.
- Have third-party testing for potency and sterility.
- Hold accreditation from organizations such as the Pharmacy Compounding Accreditation Board (PCAB).
Providers should also keep communication open with the pharmacy. Confirm how the medication is prepared, stored, and shipped. This helps reduce risks and ensures patients receive consistent care.
Communication and Patient Education
Patients often find information online that may be confusing or incomplete. Providers should take time to explain clearly:
- How compounded tirzepatide is different from Mounjaro® or Zepbound®.
- Why careful dosing and monitoring are important.
- What side effects to expect and when to seek help.
Using simple, direct language can prevent misunderstandings. Giving patients written instructions, along with verbal guidance, makes it easier for them to follow the treatment plan.
Providers should approach compounded tirzepatide with caution and structure. By following clear steps—risk assessment, informed consent, monitoring, and communication—they can support patient needs while upholding safety and professional standards.
Conclusion
Tirzepatide is a new type of medication that has gained a lot of attention for its role in treating type 2 diabetes and for helping with weight management. Because it is effective, many people want access to it. At the same time, questions about compounding have become more common. Patients and providers are asking: Is it legal? Is it safe? Is it the same as the FDA-approved brand products such as Mounjaro® or Zepbound®? The answers are not always simple, but it is important to understand the facts before making decisions.
First, compounding means that a pharmacy makes a medication in a form that is not available from the original manufacturer. This practice can help patients who need a different strength, dosage form, or ingredient combination. Compounding is legal in the United States when done by licensed pharmacies that follow state and federal rules. However, the FDA does not test or approve compounded drugs the same way it does with brand-name products. That means there can be differences in quality, consistency, and safety.
When it comes to tirzepatide, the legal status of compounding depends on several factors. Federal law allows compounding when a medication is in shortage or unavailable. During shortages of Mounjaro® or Zepbound®, some pharmacies began to offer compounded tirzepatide to fill the gap. But this practice has limits. The FDA has warned that compounding is not meant to replace mass manufacturing when the brand medication is widely available again. Providers and patients should be aware that these rules can change, and what may be allowed today may not be allowed tomorrow.
Another key issue is safety. FDA-approved tirzepatide products have been through years of clinical trials and strict review. Every vial or pen has the same dose, purity, and quality. With compounded versions, there is no guarantee of the same level of testing. The risks include contamination, inaccurate dosing, or changes in potency if the medication is not prepared or stored correctly. These risks may be higher if a patient uses a pharmacy that does not follow high standards or if the source of the active ingredient is unclear.
Effectiveness is also an open question. While tirzepatide itself has proven effectiveness, a compounded version may not work exactly the same way. This is because small differences in formulation, stability, or how the drug is absorbed by the body can affect results. For example, a compounded injection may not have the same preservative system or device design as Mounjaro® or Zepbound®. These differences might change how the body responds, even if the active ingredient is the same.
Patients and providers can take steps to reduce risks if compounded tirzepatide is being considered. One step is to verify the pharmacy’s credentials. Accredited compounding pharmacies follow strict standards, which lowers the risk of problems. Patients should also ask their provider to closely monitor their blood sugar, weight, or side effects if they are using compounded medication. Open communication between patients, providers, and pharmacists is essential.
Cost is another factor that often comes up. Compounded tirzepatide may sometimes be offered at a lower price than brand-name products. But lower cost should not mean lower safety. In many cases, insurance will not cover compounded medications, leaving patients to pay out-of-pocket. Providers should help patients understand both the financial and medical sides of their choices.
There are also broader ethical and regulatory concerns. On one hand, compounded tirzepatide may help patients who otherwise would have no access due to shortages or high costs. On the other hand, if compounded products are widely used when brand-name versions are available, it could create risks for patients and challenges for regulators. The FDA’s role is to balance access with safety, and this balance continues to evolve.
For providers, the responsibility is to guide patients with accurate information and careful judgment. That includes documenting why a compounded product is being used, explaining the risks, and making sure patients are monitored. For patients, the responsibility is to ask questions, seek care from trusted professionals, and report any problems or side effects right away.
In summary, the topic of compounded tirzepatide is complex. It involves medical science, pharmacy practice, law, and patient needs. The most important point is that compounded tirzepatide is not the same as an FDA-approved brand product. It may be legal under certain conditions, but it carries unique risks that require careful oversight. Patients and providers must work together to weigh the benefits and risks, and decisions should always be guided by safety and medical supervision.
As the supply of tirzepatide products changes and as the FDA updates its rules, the situation may shift. What stays the same is the need for clear communication, careful decision-making, and ongoing monitoring. By staying informed and cautious, patients and providers can make the best possible choices when it comes to tirzepatide and compounding.
Research Citations
U.S. Food and Drug Administration. (2025, April 28). FDA clarifies policies for compounders as national GLP-1 supply begins to stabilize. Silver Spring, MD: FDA.
U.S. Food and Drug Administration. (2025, September 5). FDA’s concerns with unapproved GLP-1 drugs used for weight loss. Silver Spring, MD: FDA.
Kosko, R., & Jensen, V. E. (2024, December 19). Resolution of tirzepatide injection product shortage and supply status (FDA memorandum). U.S. Food and Drug Administration.
U.S. Food and Drug Administration. (2024, November 15). Compounding and the FDA: Questions and answers. Silver Spring, MD: FDA.
McDermott Will & Emery. (2025, June 13). Court backs FDA in tirzepatide compounding case. Washington, DC: McDermott Will & Emery.
Buchanan Ingersoll & Rooney PC. (2025, January 27). Tirzepatide, FDA, and compounding: Understanding the current landscape. Philadelphia, PA: Buchanan Ingersoll & Rooney.
Eli Lilly and Company. (2023, September 19). Lilly statement on Mounjaro (tirzepatide) compounding litigation. Indianapolis, IN: Eli Lilly and Company.
Eli Lilly and Company. (2024, May 14). Lilly update on Mounjaro and Zepbound (tirzepatide) compounding. Indianapolis, IN: Eli Lilly and Company.
Nevada State Board of Pharmacy. (2025, June 2). Notice to compounding pharmacies regarding tirzepatide and semaglutide. Carson City, NV: Nevada State Board of Pharmacy.
University of Illinois Chicago, Drug Information Group. (2025, August 2). What are the safety concerns regarding compounded GLP-1 receptor agonists? Chicago, IL: University of Illinois Chicago.
Questions and Answers: Tirzepatide Can Be Compounded
Yes, tirzepatide can be compounded by certain licensed compounding pharmacies, but only when it meets regulatory requirements such as being listed on the FDA drug shortage list.
Compounding may be considered if there is a shortage of the commercially available product, or if a patient requires a customized formulation not offered by the manufacturer.
No, compounded tirzepatide is not FDA-approved. While it may contain the same active ingredient, its quality, safety, and effectiveness are not guaranteed in the same way as the branded products.
Yes. Potential risks include variability in dosing, contamination, or reduced effectiveness due to lack of standardized manufacturing oversight compared to FDA-approved medications.
Yes, physicians may legally prescribe compounded tirzepatide if it is clinically appropriate and meets FDA conditions, such as during a drug shortage.
Patients should ensure their medication is dispensed by a state-licensed and reputable compounding pharmacy, ideally one accredited by organizations like the Pharmacy Compounding Accreditation Board (PCAB).
Often, insurance does not cover compounded medications, including tirzepatide, meaning patients may need to pay out-of-pocket.
Compounding pharmacies may prepare tirzepatide in injectable formulations, typically in vials or prefilled syringes, but this varies depending on the pharmacy’s capabilities.
The FDA has cautioned that some compounded tirzepatide products may not meet safety or quality standards, and that consumers should be cautious when obtaining them from unverified sources.
No, patients should use the FDA-approved version whenever possible. Compounded tirzepatide should only be considered when the commercial product is unavailable or when a patient requires a specialized formulation.