Table of Contents
Introduction
Semaglutide is a medicine that helps lower blood sugar in people with type 2 diabetes. It is also used to help people lose weight. Over the last few years, it has become more popular for both of these reasons. Doctors prescribe it under brand names like Ozempic, Wegovy, and Rybelsus. As more people use semaglutide, it is important to study its long-term safety, especially in certain groups of people who may have higher health risks. One group that may need special attention includes women who are at risk for ovarian cancer.
Ovarian cancer is a serious disease that starts in the ovaries, which are part of a woman’s reproductive system. It is often called a “silent” cancer because it may not show clear signs until it is at an advanced stage. Some women are more likely to get ovarian cancer because of their genes, family history, or other health factors. These women are sometimes placed under close medical care to help find cancer early or even prevent it. Because these women already face a higher risk, doctors and researchers want to know if any new medicine they take could make that risk worse. This includes weight-loss drugs like semaglutide.
Women at risk for ovarian cancer often take steps to stay as healthy as possible. Some choose to have regular checkups and cancer screening. Others may have surgery to remove the ovaries or fallopian tubes before cancer ever forms. Many also try to manage their weight, blood sugar, and hormone levels, because these may affect cancer risk too. Since semaglutide helps with weight loss and blood sugar control, it may seem like a good option for some of these women. But before it is widely used in this group, experts want to make sure that it does not raise the chance of developing ovarian cancer.
Semaglutide works by copying a natural hormone called GLP-1. This hormone helps control blood sugar by telling the body to release insulin. It also slows down the emptying of the stomach and helps people feel full after eating. These effects help with both diabetes and weight loss. But hormones in the body often affect more than one system. Some researchers have asked if semaglutide’s actions might also change how cells grow or die, especially in parts of the body like the ovaries.
So far, semaglutide has not been clearly linked to any type of cancer in people. Large studies have not shown a strong connection between the drug and cancer in general. But because ovarian cancer is rare and takes years to develop, longer studies in special groups of people may be needed. In lab tests and animal studies, there have been some signals that GLP-1 drugs may have effects on how cells behave, but these results have not proven anything for humans.
There is also a question about how semaglutide interacts with genes linked to cancer risk. Some women carry changes in genes like BRCA1 or BRCA2. These gene changes can make it more likely to develop ovarian cancer. Researchers want to know if semaglutide has any special risks in women with these genetic backgrounds. At this time, there is not enough information to give a clear answer.
Because so many women are now using semaglutide, and many are of the age when cancer risk starts to rise, it is important to look closely at any possible risks. Doctors, patients, and researchers all need clear and up-to-date information to make safe choices. Even though semaglutide has helped many people manage weight and diabetes, careful research is needed to make sure it does not cause problems in women who are already at higher risk for certain diseases.
This article will look at what is known so far about semaglutide and its safety in women who may be at risk for ovarian cancer. It will review the science behind how the drug works, what studies have found about cancer risks, and whether special care is needed when using semaglutide in this group. The goal is to give clear and trusted information based on research—not on guesses—so that decisions can be made with confidence and care.
What Is Semaglutide and How Does It Work?
Semaglutide is a type of medicine known as a GLP-1 receptor agonist, which means it acts like a natural hormone in the body called glucagon-like peptide-1 (GLP-1). This hormone plays an important role in how the body handles blood sugar and appetite. Semaglutide is used to treat type 2 diabetes and obesity, and it helps lower blood sugar levels and body weight.
After a person eats, the body releases GLP-1. This hormone helps the pancreas release insulin, a hormone that lowers blood sugar. It also reduces the amount of another hormone called glucagon, which increases blood sugar. GLP-1 slows down how fast the stomach empties food and makes people feel full longer. Semaglutide copies these effects, helping people with diabetes manage their blood sugar and helping people with obesity lose weight.
Semaglutide is sold under several brand names. For example:
- Ozempic is used to treat type 2 diabetes.
- Wegovy is used to treat obesity and overweight.
- Rybelsus is a tablet version of semaglutide for type 2 diabetes.
All three contain the same active ingredient—semaglutide—but are used in slightly different ways and doses.
How Semaglutide Helps with Diabetes
In people with type 2 diabetes, the body does not use insulin properly, and this causes high blood sugar. Over time, this can lead to damage in organs like the heart, kidneys, and eyes. Semaglutide helps lower blood sugar in two main ways:
- It helps the pancreas release more insulin when blood sugar is high.
- It reduces how much sugar the liver makes by lowering glucagon levels.
Because it slows digestion, semaglutide also prevents blood sugar from rising too quickly after meals. For many people, this leads to better blood sugar control without large spikes or drops.
How Semaglutide Helps with Weight Loss
People using semaglutide often feel less hungry. This happens because the medicine affects parts of the brain that control appetite. It makes people feel full faster and stay full longer. Many people taking semaglutide eat smaller portions and snack less often. Over time, this can lead to a significant drop in weight.
In large studies, people who took semaglutide lost much more weight than those who made lifestyle changes alone. This is why doctors may prescribe it to people with obesity or overweight, especially if they also have health problems like high blood pressure or prediabetes.
How Semaglutide Is Taken
Semaglutide can be taken by injection or oral tablet:
- Injection: Ozempic and Wegovy are injected under the skin, usually once a week.
- Tablet: Rybelsus is taken by mouth once a day on an empty stomach.
Most people start on a low dose. The dose is increased slowly to lower the risk of side effects like nausea or stomach upset. This careful approach also helps the body adjust to the medicine.
Common Side Effects
Like any medicine, semaglutide can cause side effects. The most common ones are:
- Nausea
- Vomiting
- Diarrhea
- Constipation
- Headache
- Loss of appetite
These side effects are usually mild and improve over time. However, some people may stop taking semaglutide because the side effects are too uncomfortable.
There are also rare but serious risks. These include inflammation of the pancreas (pancreatitis), gallbladder problems, and in rare cases, a risk of thyroid tumors. People with a history of certain types of thyroid cancer may be told not to use semaglutide.
Use in Women
Semaglutide is commonly prescribed to women for both diabetes and weight management. Since it affects hormones like insulin and glucagon and influences appetite and weight, some doctors are studying how it might interact with other hormones in women. This is especially important for women who may already be at risk for hormone-related conditions like polycystic ovary syndrome (PCOS), thyroid disorders, or certain types of cancer.
Semaglutide does not contain estrogen or other reproductive hormones. However, it may have indirect effects on a woman’s hormonal balance by lowering insulin levels, changing fat distribution, and altering metabolism. These changes may have different effects depending on a woman’s age, weight, and health history.
Because it is still a newer drug, long-term studies in certain groups—like women at risk for ovarian cancer—are still ongoing or have not yet been done. This has led to questions about whether semaglutide could influence cancer risk in any way, especially in tissues like the ovaries that respond to hormonal and metabolic signals.
Understanding how semaglutide works helps make sense of why researchers are exploring its safety in special populations, including women at high risk for ovarian cancer.
How Common Is Ovarian Cancer and Who Is at Risk?
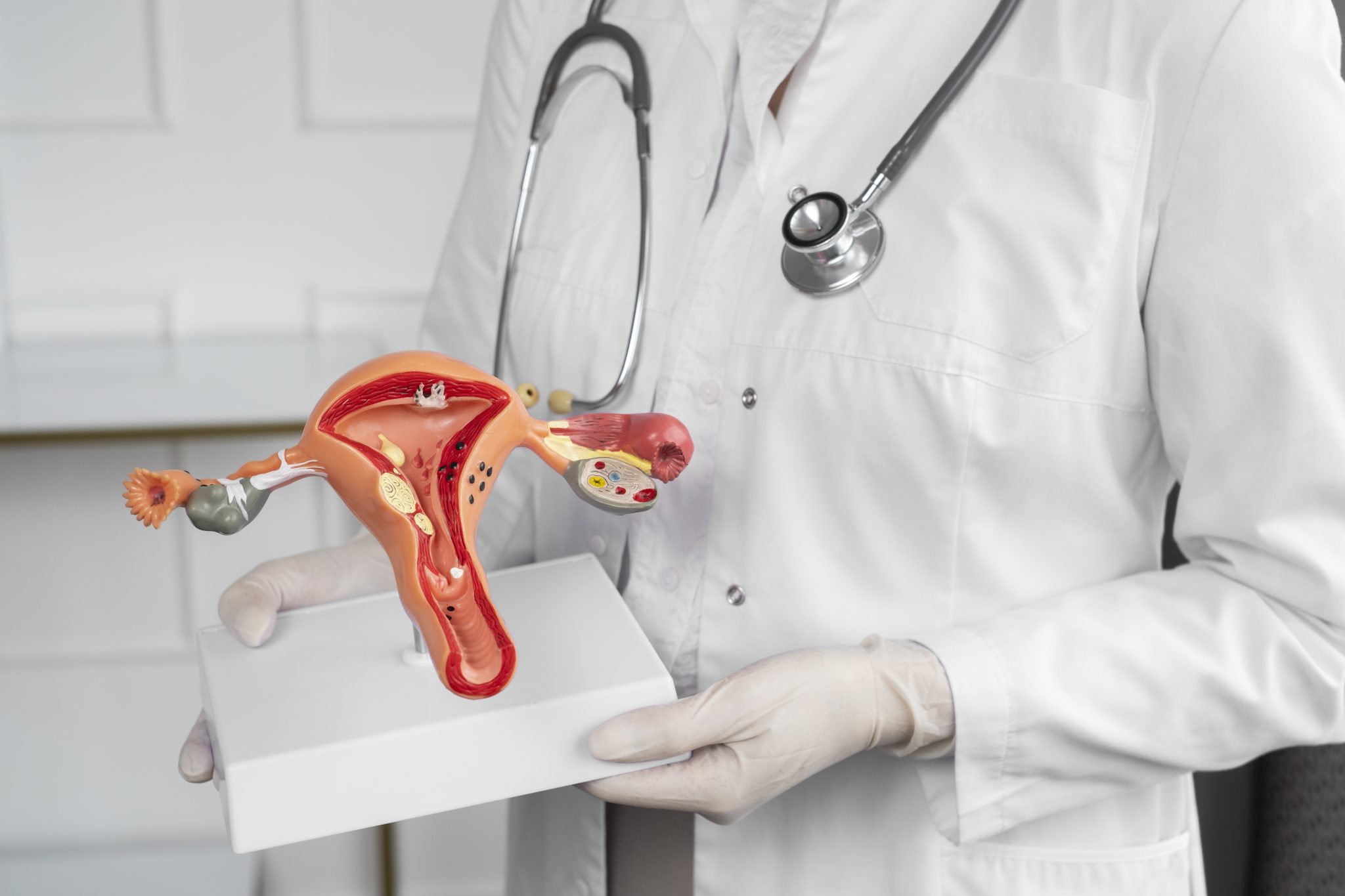
Ovarian cancer begins in the ovaries, which are the female organs that produce eggs and hormones like estrogen and progesterone. This type of cancer can be hard to detect early because its symptoms are not always clear. Even though it is not the most common cancer in women, it causes more deaths than most other cancers of the female reproductive system.
Each year, about 19,000 women in the United States are diagnosed with ovarian cancer. It is the fifth leading cause of cancer death among women. Most cases are found after the cancer has already spread, which makes treatment more difficult and lowers the chance of survival.
How Age Affects Risk
Age is one of the most important risk factors for ovarian cancer. The chance of getting this cancer increases with age. Most cases happen in women who are over 50 years old, especially between the ages of 55 and 64. It is rare in younger women, but not impossible.
As women age, changes in hormone levels and cell function in the ovaries may make it more likely for cancer to develop.
Family History and Inherited Risk
A strong family history of ovarian or breast cancer increases the chance of getting ovarian cancer. Women who have a mother, sister, or daughter with these cancers are at higher risk. This is often due to inherited gene changes passed down through families.
The BRCA1 and BRCA2 genes are the most well-known. These genes help repair damaged DNA. When they do not work properly, abnormal cells can grow more easily.
- Women with a BRCA1 mutation have a 35% to 70% lifetime risk of ovarian cancer.
- Women with a BRCA2 mutation have a 10% to 30% lifetime risk.
Another inherited condition, Lynch syndrome, also raises the risk. It affects the body’s ability to fix DNA mistakes and is linked to ovarian, colon, and other cancers.
Hormonal and Reproductive Factors
The number of times a woman ovulates (releases an egg) during her life can affect ovarian cancer risk. More ovulation may increase the chance of abnormal cell changes.
Factors that increase risk:
- Starting periods before age 12
- Going through menopause after age 50
- Never being pregnant
- Having a first pregnancy after age 35
Factors that lower risk:
- Being pregnant (especially more than once)
- Breastfeeding
- Using birth control pills
Birth control pills stop ovulation for a time, which may help protect the ovaries.
Other Medical Conditions
Some health conditions can also raise the risk of ovarian cancer.
- Obesity increases inflammation and hormone levels, which may affect the ovaries.
- Endometriosis is a condition where tissue like the uterine lining grows outside the uterus, sometimes on the ovaries. It may slightly increase the chance of certain ovarian cancers.
Environmental and Lifestyle Factors
The role of lifestyle and environment is not fully clear, but some studies have looked at these links:
- Talcum powder used in the genital area has been studied as a possible cause, but results are mixed.
- Smoking and alcohol do not have strong links to ovarian cancer, but they do affect general health and may influence other cancer risks.
Warning Signs and Early Detection
Ovarian cancer often grows quietly. Symptoms are usually vague and can seem like other, less serious problems. These include:
- Bloating
- Belly or pelvic pain
- Trouble eating or feeling full quickly
- Needing to urinate often
Because these signs are common, many women do not get tested right away. This leads to late diagnoses, when cancer is harder to treat.
Why Knowing Risk Matters
Women at higher risk—especially those with a strong family history or known genetic mutations—may benefit from:
- Regular screening tests
- Preventive surgery to remove the ovaries and fallopian tubes
- Genetic counseling to understand their personal risk
Understanding these risk factors is important when deciding on any long-term medication. Drugs that affect hormones, inflammation, or metabolism—like semaglutide—need to be carefully reviewed for their safety in women who may already be at higher risk for ovarian cancer.
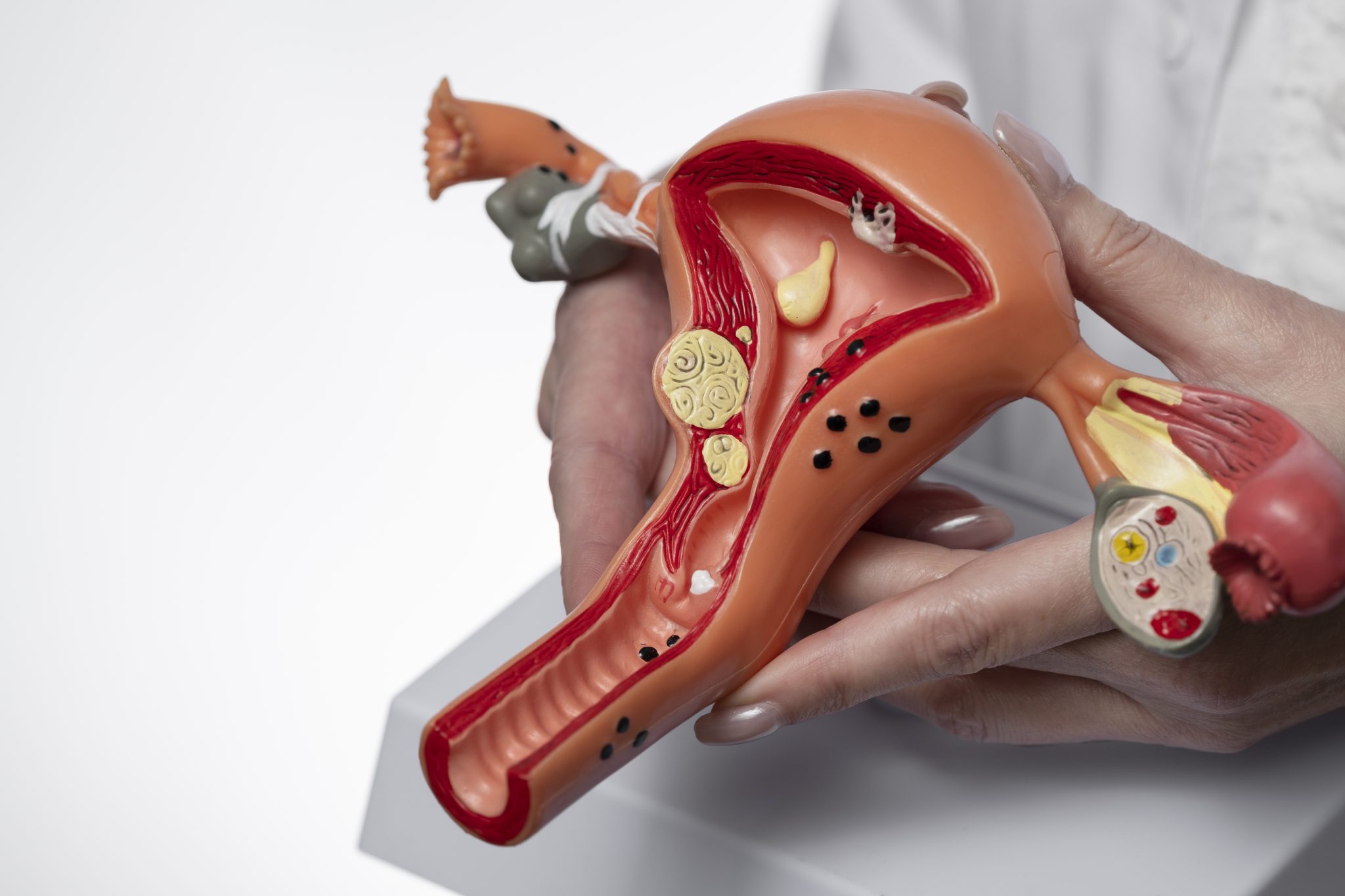
Could Semaglutide Affect Ovarian Tissue or Hormonal Function?
Semaglutide belongs to a group of medicines called GLP-1 receptor agonists. These drugs copy the action of a natural hormone in the body called glucagon-like peptide-1 (GLP-1). This hormone helps control blood sugar levels and also helps people feel full after eating. Because semaglutide works through hormone pathways, there are questions about how it might affect the ovaries or other parts of the female reproductive system. This is especially important for women who have a higher risk of ovarian cancer.
GLP-1 Receptors and Reproductive Tissues
The GLP-1 hormone works by attaching to special receptors found in different tissues in the body. These receptors are called GLP-1 receptors. Most of them are found in the pancreas, stomach, brain, and intestines. However, researchers have also found GLP-1 receptors in other areas, including the ovaries, uterus, and parts of the brain that control hormones.
This raises a question: if semaglutide can reach these areas, could it change how the ovaries work? While research is still ongoing, there is no strong evidence yet that GLP-1 receptor agonists cause changes to ovarian function in healthy women. However, some small lab studies have shown that GLP-1 can affect how cells grow or respond to stress in tissues that have these receptors.
Effects on Estrogen and Hormonal Balance
Semaglutide may have indirect effects on hormones such as estrogen. Estrogen is one of the main female sex hormones and plays a big role in the health of the ovaries, uterus, and breast tissue. In women with higher body weight or obesity, fat cells can produce extra estrogen. When semaglutide is used for weight loss, fat stores shrink, and this can lower extra estrogen levels.
Lowering excess estrogen might reduce some hormone-related cancer risks, like breast or endometrial cancer. However, the link between estrogen levels and ovarian cancer is more complex. In some cases, changing estrogen levels can influence how certain types of ovarian tumors grow. There is still not enough information to say how semaglutide-related weight loss might affect this.
Also, semaglutide helps reduce insulin levels by improving how the body uses sugar. High insulin levels are linked to increased cell growth and lower cell death, which can play a role in cancer development. By lowering insulin, semaglutide may reduce this risk in general. Still, whether this applies specifically to ovarian cancer is not yet known.
Cell Growth and Apoptosis (Cell Death)
In lab studies using animals or cancer cell lines, GLP-1 receptor agonists have shown mixed results. In some cases, they seem to reduce cancer cell growth or even increase the death of abnormal cells, a process called apoptosis. This is a good thing in cancer prevention. But in other studies, some GLP-1 medications showed possible growth signals in certain tissues.
It is important to understand that results from cell or animal studies do not always match what happens in the human body. These early studies help guide future research, but they do not prove that a medicine causes or prevents cancer in real people.
What This Means for the Ovaries
The ovaries are sensitive to changes in hormones, weight, and insulin levels. Because semaglutide changes all three, it could, in theory, have an effect. However, there is no clear evidence that semaglutide directly harms the ovaries or increases the risk of ovarian cancer. There is also no proof that it protects against ovarian cancer.
So far, large studies on semaglutide have not found any unusual patterns of ovarian problems or higher cancer risk. But most of these studies were not designed to look closely at ovarian cancer risk. More research is needed, especially in women who already have a higher risk due to family history, genetic conditions like BRCA mutations, or other medical issues.
Semaglutide may affect the body’s hormone levels, including insulin and possibly estrogen, by helping with weight loss and improving blood sugar control. It also works on GLP-1 receptors that may exist in reproductive tissues, but there is no clear proof that this leads to changes in the ovaries. Early lab research shows some effects on cell growth, but human studies do not show any clear link between semaglutide and ovarian cancer at this time. Still, more detailed studies are needed to fully understand its impact in women at higher risk for ovarian cancer.
What Does Current Research Say About Semaglutide and Cancer Risk?
Semaglutide is a medication that helps control blood sugar and reduces body weight. It is used for people with type 2 diabetes and obesity. Because it changes how the body handles insulin, sugar, and fat, researchers have looked closely at whether it could affect the risk of cancer. Many people are now asking if semaglutide might raise the chance of developing cancer, especially ovarian cancer in women at higher risk. Understanding what research shows is important for doctors and patients.
Animal and Laboratory Studies
Before medicines are tested on people, they are often studied in animals and cells. These studies help scientists learn how the drug works and if it might cause harm. Some early animal studies of GLP-1 receptor agonists, the class of drugs that includes semaglutide, showed tumors in the thyroid glands of rodents. This raised concerns about whether these medicines might lead to cancer in humans.
However, these results were found in rodents, not humans. Rodents have more GLP-1 receptors in their thyroid glands than people do. That means the way their bodies respond to semaglutide might be very different. So far, no human studies have clearly shown the same type of thyroid cancer risk. There is no strong evidence that semaglutide causes cancer in the ovaries or other parts of the female reproductive system in animal studies.
In laboratory studies using human cells, scientists tested how GLP-1 receptor agonists like semaglutide affect cancer cells. Some studies found that these drugs might slow the growth of certain cancer cells. Other studies showed no effect. These results depend on the type of cells used, the amount of drug tested, and how long the drug was applied. Lab studies can give useful clues, but they do not always match what happens in real life.
Clinical Trial Data
The strongest type of medical evidence comes from large clinical trials. These are studies where people are given either a drug or a placebo, and then their health is tracked for months or years. Several large trials have studied semaglutide in thousands of people. These include the SUSTAIN trials (for diabetes) and the STEP trials (for obesity and weight loss).
Across these trials, researchers watched closely for signs of cancer. In most of the trials, there was no clear link between semaglutide and increased risk of any type of cancer. A few participants did develop cancer, but the rates were similar in people who got semaglutide and those who got a placebo. There were no reports of increased ovarian cancer specifically in these trials.
In the SUSTAIN-6 trial, which looked at cardiovascular outcomes in people with type 2 diabetes, a small number of participants developed different types of cancer. However, the cancer cases were spread across both the semaglutide group and the placebo group. No pattern suggested that semaglutide caused these cancers. The STEP trials, which focused on weight loss, also did not show any significant increase in cancer rates.
Monitoring from Safety Agencies
The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) continue to review safety data for semaglutide. These agencies collect reports from doctors, researchers, and patients about possible side effects. So far, they have not found enough evidence to suggest that semaglutide causes ovarian cancer or other cancers in humans.
The FDA does require a warning label on GLP-1 receptor agonists about a possible risk of a rare thyroid cancer called medullary thyroid carcinoma (MTC). This warning is based on the rodent studies mentioned earlier. However, this type of cancer is very rare in people, and no clear cases have been linked to semaglutide in large human trials.
Both the FDA and EMA continue to ask for long-term follow-up studies. These studies will help determine if any cancer risk appears after many years of use, especially in people who are at higher risk due to family history or genetic factors.
So far, the best available research does not show a link between semaglutide and an increased risk of ovarian cancer. Animal studies raised early concerns, but these were not confirmed in human trials. Laboratory tests have mixed results and do not give a clear answer. Large clinical trials involving thousands of people have not shown higher cancer rates in people taking semaglutide. Safety agencies continue to watch for any warning signs, but they have not found any strong reason for concern at this time.
More research is needed, especially studies focused on women who have a higher risk of ovarian cancer. Until then, semaglutide is considered safe for most people based on the evidence available today.
Has Semaglutide Been Linked to Ovarian Cancer?
Semaglutide is a medicine used to treat type 2 diabetes and obesity. It helps control blood sugar and supports weight loss by mimicking a hormone called GLP-1 (glucagon-like peptide-1). As its use becomes more common, some people have raised concerns about whether semaglutide could be linked to cancer, especially in women who are at higher risk for ovarian cancer. So far, there is no strong evidence that directly links semaglutide to ovarian cancer. However, researchers and doctors are still looking closely at possible connections.
Reports of Cancer Cases with Semaglutide Use
Some studies have looked into whether semaglutide, or other medicines like it, might be related to any kind of cancer. In clinical trials where thousands of people took semaglutide for several months or years, the number of cancer cases reported was small. These cases included different types of cancer, but ovarian cancer was very rare. For example, in the STEP and SUSTAIN trials—large studies that tested semaglutide in people with diabetes or obesity—there were only a few cancer cases reported overall, and none clearly connected to semaglutide.
Sometimes, when a new medicine is approved, doctors and researchers keep track of side effects through a system called pharmacovigilance. This system collects reports from healthcare providers and patients about problems that may be related to medicines. A few of these reports have mentioned cancer in people taking GLP-1 receptor agonists like semaglutide. However, these reports do not prove that the medicine caused the cancer. Many people who use semaglutide also have other risk factors, such as obesity, age, or a family history of cancer, which could explain the cases.
Looking at Population Studies
Some researchers have looked at large groups of people to see if there is a higher risk of cancer in those taking GLP-1 medications. A few studies have suggested a possible increase in thyroid cancer, especially a rare type called medullary thyroid carcinoma, but the results have not been clear or consistent. There has not been a signal showing that semaglutide increases the risk of ovarian cancer in these types of population studies.
A 2022 safety review of GLP-1 receptor agonists—including semaglutide—found no strong connection between these medicines and ovarian or other reproductive cancers. This review included data from clinical trials, safety monitoring systems, and insurance claims databases. It concluded that there is currently no proof that semaglutide raises the risk of ovarian cancer.
Important Limitations in the Research
Even though no clear link has been found, some important limitations exist in current studies. First, ovarian cancer is not very common, which makes it hard to study in short-term trials. Many trials last only one or two years, but cancer can take longer to develop. Second, most of the people in these studies were not already at high risk for ovarian cancer. That means researchers still don’t know if semaglutide could have a different effect in women who carry BRCA1 or BRCA2 gene mutations, or those with a strong family history of ovarian cancer.
Another issue is that the way ovarian cancer starts is still not fully understood. It is possible that certain medicines could affect hormone levels, metabolism, or inflammation in the body—factors that could play a role in cancer risk. So far, studies have not shown that semaglutide changes the ovaries in ways that increase the risk of cancer, but more research is needed.
Coincidence vs. True Risk
When someone develops cancer while using a medicine, it can be hard to tell whether the drug played a role or if it was just a coincidence. Many people who use semaglutide are already older, have diabetes, or are overweight—factors that are already linked to higher cancer risk. This makes it more complicated to figure out if semaglutide has any direct effect.
Right now, no studies have proven a direct cause-and-effect relationship between semaglutide and ovarian cancer. Experts recommend watching for any new information as more people use the medicine and more long-term data becomes available.
There is currently no strong evidence that semaglutide causes ovarian cancer. A few cases of cancer have been reported in people using semaglutide, but these cases are rare and have not shown a clear link. Ongoing research and safety monitoring are important, especially for women at higher risk for ovarian cancer. Until more data becomes available, doctors continue to weigh the known benefits of semaglutide against any possible risks, based on each patient’s health history.

Does Semaglutide Interact with BRCA Mutations or Genetic Predisposition?
Women with a family history of ovarian cancer or a known genetic mutation, such as BRCA1 or BRCA2, are at much higher risk of developing ovarian cancer. These mutations affect how cells repair damaged DNA. If the damage is not fixed, cells can grow uncontrollably and form tumors. Because semaglutide changes how hormones work in the body and affects weight and metabolism, there are important questions about whether it could also impact cancer risk in women with these genetic conditions.
Understanding BRCA Mutations and Cancer Risk
BRCA1 and BRCA2 are genes that help protect the body by repairing DNA. When these genes are working properly, they prevent cells from turning into cancer. But if someone inherits a harmful mutation in one of these genes, their cells may not repair DNA well. This can lead to cancer, especially breast and ovarian cancer. Women with BRCA mutations have a much higher lifetime risk of ovarian cancer than women without these mutations.
BRCA mutations are most common in people of Ashkenazi Jewish descent, but they can occur in any population. Not all women with these mutations will develop cancer, but many are advised to undergo regular screenings, take preventive medications, or even have surgery to remove the ovaries before cancer starts.
How Semaglutide Works in the Body
Semaglutide belongs to a group of drugs called GLP-1 receptor agonists. These medicines copy the effect of a hormone called GLP-1, which helps control blood sugar and appetite. Semaglutide slows digestion, reduces hunger, and helps people lose weight. It also affects insulin and glucagon, two hormones that manage how the body uses sugar.
The drug does not directly affect DNA repair. It does not target the BRCA genes or their pathways. However, it changes many systems in the body, including hormones, inflammation, and metabolism. Because of these changes, scientists want to know if semaglutide could have an effect—positive or negative—on cancer risk in women who are already at high genetic risk.
Research on Semaglutide and Genetic Cancer Risk
So far, no published studies have directly looked at semaglutide in women with BRCA1 or BRCA2 mutations. Most of the clinical trials for semaglutide focus on type 2 diabetes or obesity in the general population. These trials include both men and women, but they do not separate the results by BRCA status or family history of cancer. This means there is no strong evidence to say whether semaglutide is safe, risky, or neutral for women with a genetic risk of ovarian cancer.
In animal studies and laboratory tests, semaglutide has not shown any clear link to ovarian cancer. However, these models usually do not have BRCA mutations. Because of this, the results cannot fully explain what might happen in humans with a genetic predisposition.
Some studies have looked at the general link between GLP-1 receptor agonists and cancer, including thyroid and pancreatic cancers. These results are mixed. Some show no increased risk, while others raise questions. But ovarian cancer has not been a main focus in any of these studies, especially not in high-risk genetic groups.
Theoretical Considerations
Although there is no direct evidence, scientists can still look at how semaglutide might affect cancer processes in theory. One area of concern is insulin and related hormones. High levels of insulin and insulin-like growth factors have been linked to cancer development. Since semaglutide lowers insulin levels, it might actually reduce cancer risk. This could be helpful for women with BRCA mutations, but it has not been proven.
Another area to consider is inflammation. Chronic inflammation can increase the risk of cancer. Semaglutide has been shown to reduce inflammation in some people. This might be another way it could protect against cancer, although again, this is only a theory.
Weight loss is also important. Obesity increases the risk of many cancers, including ovarian cancer. Since semaglutide leads to weight loss, it might lower cancer risk in that way. But whether this benefit outweighs other possible effects in women with BRCA mutations is still unknown.
Need for More Research
At this time, there are no strong data to suggest that semaglutide is harmful or helpful for women with BRCA mutations. The lack of studies means there are still many questions. Health professionals must be cautious and look at each patient’s full medical history before prescribing semaglutide to women with a genetic risk of ovarian cancer.
More research is needed to understand if semaglutide interacts with the BRCA pathways or changes the cancer risk in women with these mutations. Future studies should include women with known genetic risks and follow them over many years to see how semaglutide affects their health.
Until more information is available, the decision to use semaglutide in this population should involve careful discussion between the doctor and patient, considering personal and family cancer history, current health status, and treatment goals.
Can Semaglutide Influence Cancer Screening or Mask Symptoms?
Semaglutide is a medicine that helps with weight loss and blood sugar control. It is used by many women, including those who may be at higher risk for ovarian cancer. Because semaglutide changes how the body works in some ways, it is important to understand how it might affect cancer screening and symptom awareness, especially for ovarian cancer.
Weight Loss Can Change Biomarker Levels
One important screening test for ovarian cancer is the CA-125 blood test. This test measures a protein that can be higher in women with ovarian cancer. However, CA-125 is not a perfect test. Many other conditions—such as endometriosis, menstruation, or even liver problems—can cause high levels of CA-125. Weight loss and changes in metabolism may also affect this test.
Semaglutide causes weight loss by slowing digestion, reducing appetite, and helping the body use insulin better. As body fat decreases, hormones and proteins in the blood may change. Some small studies have suggested that CA-125 levels can decrease with weight loss, even if cancer is not present. This could make it harder to use the CA-125 test for early signs of ovarian cancer, especially in women who are overweight or obese. If the CA-125 level goes down due to weight loss, it may give a false sense of safety.
Imaging May Be Harder to Interpret
Doctors also use imaging, like ultrasounds or CT scans, to look for signs of ovarian cancer. In women who lose a lot of weight quickly, changes in body fat can affect how organs look on scans. Less fat around the ovaries or abdomen might make tumors easier to see in some cases. But in other cases, it might lead to confusion if organs shift slightly or if new tissue changes appear.
For example, rapid weight loss may cause the ovaries to appear smaller or change position. This can sometimes be misread on imaging studies. Also, weight loss might uncover old scars or cysts that were not seen before, leading to more tests or biopsies. These changes do not mean cancer is present, but they can affect how screening results are understood.
Symptoms of Ovarian Cancer May Be Overlooked
Ovarian cancer is often called a “silent” disease because symptoms can be vague and easy to ignore. Common symptoms include bloating, feeling full quickly, pelvic pain, and changes in bowel habits. These signs are often mistaken for less serious problems, especially in the early stages of the disease.
Semaglutide can cause side effects like nausea, bloating, and upset stomach. These symptoms overlap with early symptoms of ovarian cancer. A woman taking semaglutide might think her stomach pain or feeling of fullness is just a side effect of the medicine. This could delay her from reporting new or worsening symptoms to a doctor.
In addition, because semaglutide helps with weight loss, a drop in weight may be seen as a good thing—even if it is caused by something more serious. Unplanned or extreme weight loss is also a sign of cancer, but in someone taking semaglutide, it might not be investigated right away.
Regular Monitoring Is Important
For women at high risk of ovarian cancer, such as those with a strong family history or BRCA mutations, regular monitoring is critical. This often includes blood tests and pelvic imaging. When using semaglutide, doctors may need to adjust how often these tests are done or how results are interpreted.
It is important that healthcare providers are aware of all the medicines their patients are taking, including semaglutide. This helps ensure that test results are not misunderstood. In some cases, extra screening or closer follow-up might be needed to make sure no warning signs are missed.
Semaglutide can affect how the body looks and feels, which may influence how ovarian cancer is screened and detected. Weight loss may lower CA-125 levels and change how imaging appears. Digestive side effects can also look like early cancer symptoms. Because of this, women taking semaglutide—especially those at risk for ovarian cancer—should stay in close contact with their healthcare team and report any new or unusual symptoms. Regular screenings and clear communication can help make sure important signs are not missed.

Is Semaglutide Safe for Women Undergoing Surveillance or Preventive Surgery?
Women who are at high risk for ovarian cancer often follow special medical plans. These plans may include regular check-ups with imaging or blood tests, genetic counseling, or even preventive surgeries. Some of these women also have conditions like obesity or type 2 diabetes. For those reasons, semaglutide—a medicine that helps with weight loss and blood sugar control—might be considered as part of their care. However, it is important to look at how semaglutide may affect women going through cancer surveillance or planning surgery.
Preventive Surgery and Risk Reduction
Many women at high risk for ovarian cancer consider a type of surgery called risk-reducing salpingo-oophorectomy. This means removing the ovaries and fallopian tubes before cancer has a chance to grow. This decision is usually based on family history, BRCA gene mutations, or other genetic markers. Doctors often recommend this surgery between the ages of 35 and 45, depending on family planning and health status.
Weight and general health can affect surgery. High body weight is linked to longer surgeries, higher infection risk, and slower recovery. For this reason, weight loss before surgery can be helpful. Semaglutide may help these women reach a healthier weight before their procedure. Studies show semaglutide can lead to significant weight loss over a few months, especially when paired with diet and exercise. This may make surgery safer and recovery smoother.
Timing and Weight Loss Goals
If a woman is planning to use semaglutide before surgery, timing is important. The weight loss effect of semaglutide takes time—often three to six months for the full benefit. Doctors may recommend starting semaglutide well before the planned surgery date. However, sudden weight loss too close to surgery might increase certain risks, like poor wound healing or changes in blood pressure.
Also, semaglutide can sometimes cause nausea, vomiting, or loss of appetite. These side effects could affect nutrition, which is important for healing after surgery. A woman preparing for surgery needs enough protein, vitamins, and minerals to recover well. If semaglutide makes eating difficult, it may be necessary to adjust the dose or stop the medication in advance.
Doctors must work closely with patients to find the right time to stop semaglutide before surgery. Guidelines suggest stopping GLP-1 receptor agonists like semaglutide at least one week before surgery due to concerns about stomach emptying and risk of aspiration under anesthesia.
Use During Surveillance and Follow-Up
Some women do not choose surgery right away. Instead, they may follow a high-risk screening program. This may include regular pelvic exams, blood tests like CA-125, and imaging such as ultrasounds or MRIs. These women also might take semaglutide for weight loss or diabetes care.
Using semaglutide during this period is not known to interfere with the accuracy of screening tests. There is no evidence that semaglutide affects CA-125 levels directly. However, doctors may need to watch for other symptoms. Semaglutide can cause stomach discomfort, which might feel similar to symptoms of ovarian problems. For example, bloating, fullness, or cramping are common side effects of semaglutide, but they can also be warning signs of ovarian cancer. It is important that health care providers understand these side effects and do not mistake them for cancer symptoms—or miss signs of real concern.
Safety in the Postoperative Period
After surgery, many women continue to manage weight or diabetes. Restarting semaglutide after recovery may help with long-term health goals. However, it should only be resumed once the person is eating well and healing without problems. Starting too early could lead to nausea or vomiting, which may stress the body while it heals.
Doctors usually recommend restarting semaglutide several weeks after surgery, depending on how recovery is going. It is also important to monitor hydration, nutrition, and blood sugar during this time, especially in people with diabetes.
Coordinated Care Matters
Women at high risk for ovarian cancer often see several types of doctors: gynecologists, oncologists, endocrinologists, and primary care providers. When semaglutide is being used, clear communication between these doctors is important. Together, they can make sure the medicine is used in a way that supports cancer prevention and overall health.
Using semaglutide before or after preventive surgery, or during active surveillance, may be safe and helpful—but it must be carefully planned. Each woman’s case is different, and choices about semaglutide use should always be made with input from her full medical team.
What Are the Broader Safety Concerns in High-Risk Female Populations?
Semaglutide is often prescribed to help manage type 2 diabetes and obesity. Many women who take semaglutide may already have other health risks, including the risk of cancer. For women who are already at high risk for ovarian cancer, it’s important to understand how semaglutide may affect their overall health. This includes learning whether semaglutide could raise the risk of other types of cancer or cause problems with other parts of the body. This section looks closely at these broader safety concerns.
Cancer Risk in Women Taking Semaglutide
One of the biggest safety questions about semaglutide is whether it may raise the risk of cancer. So far, studies have not shown a clear link between semaglutide and ovarian cancer. However, researchers have been looking at other cancers that might be affected by semaglutide or other GLP-1 receptor agonists. These include thyroid cancer, breast cancer, and pancreatic cancer.
Animal studies have shown that semaglutide can increase the risk of thyroid C-cell tumors in rats. Because of this, the U.S. Food and Drug Administration (FDA) placed a black box warning on semaglutide. This is the strongest type of warning that the FDA gives. It means the drug should not be used by people with a personal or family history of medullary thyroid cancer or a condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). However, these tumors have not been seen in human studies in the same way they were seen in animals.
For breast cancer and pancreatic cancer, the data is less clear. Some early studies suggested a possible link between GLP-1 medications and an increased risk of these cancers. Later research has not confirmed this risk. More large and long-term studies are still needed to fully understand these possible connections.
For women at risk of ovarian cancer, understanding the full safety picture is important. If a woman already has a genetic mutation that raises her risk for cancer—like a BRCA mutation—it’s important that her healthcare team considers all possible risks and benefits of semaglutide.
Hormonal and Metabolic Changes
Semaglutide works by mimicking a hormone called GLP-1. This hormone affects insulin, appetite, and digestion. Because hormones play a large role in the body—especially in women—it’s important to know whether these changes could affect cancer risk.
Some types of cancer, including ovarian and breast cancer, are affected by hormone levels in the body. Semaglutide causes weight loss, which often leads to lower levels of estrogen in fat tissue. This could be helpful in some cases, since higher estrogen levels have been linked to some cancers. But weight loss and changes in insulin levels may also affect other hormone systems. It is not fully known how these changes may impact long-term cancer risk, especially in high-risk women.
General Safety Warnings for Women
Beyond cancer risk, semaglutide has other safety warnings. Common side effects include nausea, vomiting, diarrhea, and stomach pain. These symptoms can be more serious in some people, especially if they have other medical problems. In rare cases, semaglutide may cause pancreatitis (inflammation of the pancreas) or kidney problems.
For women with a high risk of ovarian cancer, it’s important to think about whether these side effects might delay or complicate care. For example, if a woman needs surgery to remove her ovaries as a preventive step, she may need to stop semaglutide before the procedure. The effects of semaglutide on appetite and weight may also affect how the body heals after surgery.
There may also be concerns if a woman is planning to become pregnant. Semaglutide is not recommended during pregnancy. Women of childbearing age who are at high risk for ovarian cancer may need careful planning if they are taking semaglutide and also considering future fertility treatments, genetic testing, or surgery.
Making Safe Choices With Medical Teams
The decision to use semaglutide should always be made with the help of a healthcare team. This team may include a primary care doctor, a gynecologist, an oncologist, and a genetic counselor. For women who are already dealing with cancer risk, it is especially important that all health providers are aware of medications that could impact overall safety.
Healthcare providers will often weigh the benefits of weight loss and blood sugar control against the possible risks. This is called a risk–benefit assessment. In most cases, the benefits of semaglutide are clear, especially in helping to lower blood sugar and reduce weight. Both of these can help reduce the risk of many diseases, including some types of cancer. But for women with a strong family history of cancer or a known genetic risk, extra care should be taken.
Understanding how semaglutide works in the body—and how it may affect women with unique risk factors—is key to using it safely. Right now, there is no strong evidence that semaglutide causes cancer in humans, but research is ongoing. Until more is known, healthcare teams must use caution and work closely with patients to make the best choices for long-term health.
What Do Guidelines and Experts Say About Semaglutide Use in Cancer Risk Populations?
Medical groups and health organizations provide guidance on how to use semaglutide safely. Most focus on diabetes and obesity. However, some also give advice on using semaglutide in people who may have a higher risk of cancer, including women who are more likely to develop ovarian cancer.
Recommendations from Diabetes and Endocrine Societies
The American Diabetes Association (ADA) recommends semaglutide for people with type 2 diabetes, especially when weight loss is needed or when there is a risk of heart disease. The ADA does not say that semaglutide should be avoided in people with a family history of cancer or with cancer risk genes like BRCA1 or BRCA2. However, it does suggest using caution in people with a personal history of certain cancers, especially thyroid cancer.
The American Association of Clinical Endocrinology (AACE) supports using GLP-1 receptor agonists, including semaglutide, for managing type 2 diabetes and obesity. The AACE does not list ovarian cancer risk as a reason to avoid these drugs. Still, AACE emphasizes that doctors should think carefully about the patient’s full medical history before prescribing any medicine.
Although these guidelines do not directly address women at risk of ovarian cancer, they stress the importance of personalized care. If a woman has a strong family history of cancer, doctors are encouraged to consider that when choosing treatment options.
Guidance from Cancer Organizations
The American Society of Clinical Oncology (ASCO) has not made a specific statement about semaglutide and ovarian cancer. However, ASCO and other cancer groups often warn that drugs with hormonal or metabolic effects should be studied carefully in people with cancer risk. Since semaglutide changes how the body handles insulin and fat, researchers and doctors watch for possible links to cancer development, even if no strong link has been found yet.
Some cancer experts say that drugs like semaglutide should be studied more in people who have cancer or who are likely to get it. This includes women with BRCA mutations or a family history of ovarian cancer. Right now, clinical trials for semaglutide often do not include these high-risk groups. As a result, doctors have little information to guide decisions in these situations.
Regulatory Warnings from the FDA and EMA
The U.S. Food and Drug Administration (FDA) approved semaglutide for diabetes in 2017 and for weight loss in 2021. The FDA includes a warning in the drug label about the risk of thyroid C-cell tumors based on animal studies. So far, no link between semaglutide and human ovarian cancer has been proven. The FDA does not require any special cancer screening for women who take semaglutide.
The European Medicines Agency (EMA) also reviewed semaglutide’s safety before approval. The EMA found no clear link to ovarian cancer or other female-specific cancers, but it agreed that further long-term data is needed. Both the FDA and EMA continue to monitor for safety signals through adverse event reporting systems. So far, they have not found strong evidence of increased cancer risk in humans, including ovarian cancer.
Clinical Practice and Real-World Caution
Doctors and health care teams often must decide how to use semaglutide in people with complex medical histories. In real-world practice, some physicians choose to avoid semaglutide in patients who have active cancer or a recent history of cancer, even though there is no direct rule against it. This is a precautionary approach. In contrast, some doctors may use semaglutide if they believe the benefits, like weight loss or better blood sugar control, outweigh any possible risks.
For women who are at higher risk for ovarian cancer, such as those with BRCA mutations, there are no official rules or guidelines about semaglutide. Most doctors will look at each case individually. They may involve specialists, such as gynecologic oncologists, when deciding what medicine is safest.
Some medical centers have started research programs to study how weight-loss medications like semaglutide affect cancer risk. These studies are still ongoing, and results are not yet available.
Gaps in Research and the Need for Clearer Guidelines
There is a clear need for more research on semaglutide use in people at risk for cancer. Right now, there are no specific guidelines from major health organizations about semaglutide use in women who may develop ovarian cancer. Until more data is available, doctors must rely on general safety data, expert judgment, and close patient monitoring.
Most current guidance says semaglutide is safe for most people. But extra caution may be needed for those with a family history of ovarian cancer or with cancer-related gene mutations. More studies are needed to help doctors make better decisions for these high-risk groups. Clearer guidelines will help improve care and reduce uncertainty for both patients and healthcare providers.
Conclusion
Semaglutide is a medicine that helps lower blood sugar and supports weight loss. It works by copying the hormone GLP-1, which affects how the body handles food, insulin, and appetite. Many people with type 2 diabetes and obesity use semaglutide to improve their health. However, some women who use this drug may also have a higher risk of developing ovarian cancer, either because of their family history, age, or certain genetic mutations like BRCA1 or BRCA2. For these women, it is important to understand whether semaglutide is safe or if it could raise the chance of cancer in any way.
There is currently no strong evidence that semaglutide causes ovarian cancer. In large studies where thousands of people took semaglutide for weight loss or diabetes, there were no clear increases in ovarian cancer cases. Most clinical trials and safety reviews have not shown a pattern of semaglutide causing new cancers in people using it. This includes trials like the SUSTAIN and STEP series. In fact, most safety concerns about semaglutide have focused on rare types of thyroid cancer in rodents, not on ovarian cancer in humans.
Some research has looked at how semaglutide affects the body’s hormones and tissues. Since it acts on the hormone GLP-1, and GLP-1 receptors may exist in some parts of the female reproductive system, scientists have studied whether it could change how ovarian cells grow or function. So far, no strong data show that semaglutide directly harms the ovaries or changes hormone levels in a way that leads to cancer. Still, more studies are needed to fully understand how this drug might interact with female reproductive organs over time.
Women with genetic risks for ovarian cancer, such as those with BRCA mutations, may worry about any drug that could influence cancer pathways. BRCA genes help repair damaged DNA, and when they don’t work properly, cancer risk goes up. There is no proof that semaglutide affects DNA repair or BRCA-related pathways. So far, it has not been shown to increase cancer risk in women with these gene mutations. However, women with BRCA mutations are often followed closely by doctors, and they may undergo preventive surgeries or frequent tests. In such cases, doctors must carefully weigh the benefits and possible risks of all medications, including semaglutide.
Semaglutide causes weight loss, which can affect how ovarian cancer is found or tracked. For example, weight changes can lower CA-125 levels, a blood marker sometimes used in ovarian cancer screening. This might make early signs of cancer harder to detect in some women. Also, weight loss can change how organs look in scans like ultrasounds or MRIs. These changes may lead to missed signs or delayed diagnoses. Health providers must stay alert to symptoms and adjust how they monitor women using semaglutide, especially if those women are being watched for cancer risk.
Women who are preparing for preventive surgery, such as removing the ovaries and fallopian tubes, may be taking semaglutide before or after surgery. There is no evidence that semaglutide interferes with surgical recovery, but any medication that affects weight or metabolism may require adjustments to surgical plans or timing. For instance, rapid weight loss before surgery may affect healing, and nausea or vomiting from semaglutide might change how a patient eats or prepares for surgery. Doctors should consider these factors when treating women at high risk.
In general, semaglutide has not been linked to a higher risk of cancer in clinical use. Still, questions remain about how it affects women who already have a higher baseline risk, like those with genetic or family histories of ovarian cancer. Current medical guidelines do not say semaglutide is unsafe for these women, but they also do not offer detailed instructions for this group. Because of this, more research is needed to study how semaglutide affects women with specific cancer risks over many years.
Doctors should continue to use careful judgment when prescribing semaglutide to women at risk of ovarian cancer. The known benefits for blood sugar control and weight loss can be very helpful, especially since obesity and diabetes are themselves risk factors for some cancers. But each case should be reviewed based on personal and family history, genetic background, and other risk factors. Regular follow-up and cancer screenings should continue while using semaglutide.
More studies are needed that focus directly on cancer risk in high-risk women taking semaglutide. These should include long-term data, hormone testing, and detailed tracking of cancer outcomes. Until more is known, semaglutide should be used with caution and close monitoring in women at risk. Understanding its full safety profile will help guide better decisions and protect women’s health in the future.
Research Citations
Nagendra, L., Bg, H., Sharma, M., & Dutta, D. (2023). Semaglutide and cancer: A systematic review and meta-analysis. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 17(9), 102834. doi:10.1016/j.dsx.2023.102834
Wang, L., Xu, R., Kaelber, D. C., & Berger, N. A. (2024). Glucagon-like peptide 1 receptor agonists and 13 obesity-associated cancers in patients with type 2 diabetes. JAMA Network Open, 7(7), e2416783. doi:10.1001/jamanetworkopen.2024.21305
Kanie, T., Mizuno, A., Takaoka, Y., Suzuki, T., Yoneoka, D., Nishikawa, Y., … Kwong, J. S. (2025). Association between glucagon-like peptide-1 receptor agonists and ovarian cancer survival: A population-based cohort study. Gynecologic Oncology, 199, 57–64. doi:10.1016/j.ygyno.2025.01.005
He, W., Yu, S., Wang, L., He, M., Cao, X., Li, Y., & Xiao, H. (2016). Exendin-4 inhibits growth and augments apoptosis of ovarian cancer cells. Molecular and Cellular Endocrinology, 436, 240–249. doi:10.1016/j.mce.2016.06.015
Kosowska, A., Gallego-Colon, E., Garczorz, W., Kłych-Ratuszny, A., Aghdam, M. R. F., Wozniak, M., … Wojnar, J. (2017). Exenatide modulates tumor–endothelial cell interactions in human ovarian cancer cells. Endocrine Connections, 6(10), 856–865. doi:10.1530/EC-17-0256
Correia, C. M., Barquín, A., & Díez, A. (2023). Novel treatments for obesity: Implications for cancer prevention and treatment. Nutrients, 15(17), 3737. doi:10.3390/nu15173737
Lin, A., Ding, Y., Li, Z., Jiang, A., Liu, Z., Wong, H. Z. H., … Luo, P. (2025). Glucagon-like peptide-1 receptor agonists and cancer risk: Advancing precision medicine through mechanistic understanding and clinical evidence. Biomarker Research, 13(1), 50. doi:10.1186/s40364-025-00765-3
Select STEP Programme Investigators. (2024). Effect of once-weekly subcutaneous semaglutide on body weight and safety outcomes: The STEP randomized clinical trial. JAMA, 325, 1414–1425. doi:10.1001/jama.2021.1414
Nagendra, L., Bg, H., Sharma, M., & Dutta, D. (2023). Semaglutide trials: Safety follow-up and cancer signals over three years. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 17(9), 102834. doi:10.1016/j.dsx.2023.102834
Wang, C., Wu, Z., Zhou, J., Cheng, B., & Huang, Y. (2025). Semaglutide, a glucagon-like peptide-1 receptor agonist, inhibits oral squamous cell carcinoma growth through p38 MAPK signaling pathway. Journal of Cancer Research and Clinical Oncology, 151(3), 103–112. doi:10.1007/s00432-025-06154-5
Questions and Answers: Semaglutide and Ovarian Cancer
Semaglutide is a GLP-1 receptor agonist used primarily for the treatment of type 2 diabetes and obesity. It mimics the hormone GLP-1 to regulate blood sugar levels and appetite.
No, semaglutide is not approved or used as a treatment for ovarian cancer. Its primary indications are for managing type 2 diabetes and chronic weight management.
As of now, there is limited direct research linking semaglutide to ovarian cancer prevention or treatment. However, studies are ongoing to explore the broader anticancer potential of GLP-1 receptor agonists.
Some observational studies suggest that GLP-1 receptor agonists may lower the risk of certain cancers, particularly those related to obesity and insulin resistance, but definitive evidence is still lacking.
There is no current evidence that semaglutide increases the risk of ovarian cancer. However, long-term safety data are still being collected.
GLP-1 receptor agonists may impact pathways involved in cell proliferation, inflammation, and insulin sensitivity, all of which are factors relevant to cancer development and progression.
Some preliminary studies suggest that certain cancer cells, including ovarian cancer cells, may express GLP-1 receptors, but this is still under investigation and varies by tumor type.
Generally, semaglutide can be used in patients with ovarian cancer if they have coexisting type 2 diabetes or obesity, but it should be prescribed under medical supervision.
Obesity is a known risk factor for ovarian cancer. By promoting weight loss and improving metabolic health, semaglutide may indirectly reduce ovarian cancer risk.
As of mid-2025, no major clinical trials have specifically tested semaglutide in ovarian cancer patients. Research is ongoing in broader oncology and metabolic health contexts.

Dr. Jay Flottman
Dr. Jay Flottmann is a physician in Panama City, FL. He received his medical degree from University of Texas Medical Branch and has been in practice 21 years. He is experienced in military medicine, an FAA medical examiner, human performance expert, and fighter pilot.
Professionally, I am a medical doctor (M.D. from the University of Texas Medical Branch at Galveston), a fighter pilot (United States Air Force trained – F-15C/F-22/AT-38C), and entrepreneur.