Table of Contents
Introduction
Rheumatoid arthritis (RA) is a long-term autoimmune disease that causes inflammation, pain, and swelling in the joints. Over time, this inflammation can damage cartilage and bone, leading to deformity, stiffness, and disability. It affects millions of people worldwide, most often women between the ages of 30 and 60. RA is not only a disease of the joints—it is a whole-body condition. People with RA often face fatigue, weight changes, and a higher risk of other health problems, such as heart disease and diabetes. Even though many effective treatments exist, such as disease-modifying antirheumatic drugs (DMARDs) and biologic agents, many patients still do not reach full remission. Some continue to experience pain and inflammation, and others develop side effects or lose response to therapy. Because of this, researchers continue to look for new ways to control inflammation and improve quality of life.
In recent years, scientists have discovered that metabolism and inflammation are closely linked. The immune system and the body’s metabolism communicate through chemical messengers. When metabolism becomes unbalanced, as in obesity or insulin resistance, it can make inflammation worse. In rheumatoid arthritis, metabolic changes—like altered glucose and fat metabolism—may make the immune system more active. This has led researchers to wonder whether drugs that improve metabolic health could also help control autoimmune inflammation.
Tirzepatide is one such drug that has gained attention. It is a new type of medication known as a “dual incretin receptor agonist.” This means it works on two hormone receptors in the body: the glucose-dependent insulinotropic polypeptide (GIP) receptor and the glucagon-like peptide-1 (GLP-1) receptor. Both of these hormones play important roles in controlling blood sugar and body weight. Tirzepatide was first approved for the treatment of type 2 diabetes, where it helps lower blood sugar levels, promotes weight loss, and improves cholesterol balance. More recently, it has also been approved for obesity treatment in people without diabetes because of its powerful effects on body weight and metabolism.
What makes tirzepatide interesting to scientists studying rheumatoid arthritis is that it seems to do more than regulate blood sugar. Studies have shown that it reduces inflammation in the body, lowers markers such as C-reactive protein (CRP), and improves overall metabolic health. Other drugs that act on the GLP-1 receptor, such as semaglutide and liraglutide, have also been shown to reduce inflammation in laboratory studies and in people with metabolic diseases. This has raised the question: could tirzepatide, with its dual action, also help reduce the inflammation seen in autoimmune conditions like rheumatoid arthritis?
This question is especially important because many people with rheumatoid arthritis also struggle with obesity or metabolic syndrome. These conditions can make RA worse and harder to treat. Obesity leads to higher levels of inflammatory cytokines—proteins that fuel the autoimmune attack in RA. Fat tissue also produces hormones called adipokines, which can increase joint inflammation. By reducing body fat and improving insulin sensitivity, tirzepatide could indirectly lower inflammation in the joints. Its potential to target both metabolism and inflammation makes it a unique candidate for study in rheumatoid arthritis.
The link between RA and metabolic health has become a major topic of research. Scientists now describe RA not just as a disease of the immune system, but as one influenced by the body’s metabolic state. This connection suggests that improving metabolism could have far-reaching benefits for controlling inflammation and protecting joints. Tirzepatide may offer a way to address both aspects at once—helping patients manage weight and reduce disease activity at the same time.
The goal of this article is to explain how tirzepatide might work in rheumatoid arthritis, review the current scientific evidence, and discuss what the future may hold. We will begin by describing what tirzepatide is and how it functions in the body. Then, we will look at the biology of rheumatoid arthritis and how inflammation develops. Next, we will explore the potential mechanisms that link tirzepatide to immune regulation, followed by a review of available research and clinical data. We will also examine ongoing studies, potential benefits, and safety considerations. Finally, we will discuss the challenges and research gaps that must be addressed before tirzepatide can be considered a real option for patients with rheumatoid arthritis.
In summary, rheumatoid arthritis remains a complex condition that requires new and better treatment strategies. Tirzepatide, though currently approved for diabetes and obesity, shows promise in its ability to influence both metabolism and inflammation—two key factors in RA. Understanding how this medication works and what evidence supports its use could open a new chapter in the management of autoimmune diseases. By connecting the dots between metabolism, immunity, and inflammation, researchers hope to uncover new ways to ease the burden of rheumatoid arthritis and improve patient outcomes in the years ahead.
What Is Tirzepatide?
Tirzepatide is a new type of medication that works in a unique way to control blood sugar and body weight. It belongs to a class of drugs called dual incretin receptor agonists. This means that it activates two natural hormones in the body — GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1). These two hormones help control how the body handles food, sugar, and energy. By working on both receptors at the same time, tirzepatide has effects that go beyond those seen with older diabetes medicines.
Dual Mechanism of Action
Most diabetes drugs act on a single pathway. For example, older medications like GLP-1 receptor agonists (such as semaglutide or liraglutide) act only on the GLP-1 receptor. Tirzepatide, however, acts on both GLP-1 and GIP receptors. This “dual action” gives it stronger effects on blood sugar control and weight loss.
Here is how it works:
- At the GLP-1 receptor:
Tirzepatide increases the amount of insulin released from the pancreas when blood sugar is high. It also slows down how fast the stomach empties food, which helps reduce hunger and control food intake. In the brain, it acts on appetite centers to make people feel full sooner and stay full longer. GLP-1 activity also lowers the release of glucagon — a hormone that raises blood sugar — which helps keep blood glucose in a healthy range. - At the GIP receptor:
GIP has its own role in controlling metabolism. It helps the body use fat more effectively and may improve how muscle and fat cells respond to insulin. Activation of GIP receptors may also enhance the positive effects of GLP-1 on weight loss and energy balance. Scientists believe that GIP may help reduce inflammation and improve insulin sensitivity in tissues throughout the body.
Together, these actions make tirzepatide a “next-generation” metabolic therapy. By targeting both incretin pathways, it offers a more complete way to manage metabolic health than older single-pathway drugs.
Approved Uses and Clinical Indications
Tirzepatide was first developed and approved for type 2 diabetes under the brand name Mounjaro®. It has also received approval in several countries, including the United States, for chronic weight management under the brand name Zepbound™.
In diabetes care, tirzepatide helps lower both fasting and post-meal blood glucose levels. In clinical studies, patients taking tirzepatide often experienced major improvements in HbA1c (a long-term measure of blood sugar control) and lost a significant amount of body weight — often more than with other available diabetes drugs. These benefits have made it one of the most discussed metabolic therapies in recent years.
For weight management, tirzepatide is approved for adults who are obese or overweight and have at least one weight-related condition such as hypertension, high cholesterol, or diabetes. It helps reduce appetite and energy intake, leading to sustained weight loss over time.
Pharmacokinetics and Dosing
Tirzepatide is given by subcutaneous injection, usually once a week. It has a long duration of action — its half-life is about five days — which allows for convenient weekly dosing. The dose is typically started low and increased gradually to improve tolerance and reduce side effects such as nausea.
Once injected, tirzepatide circulates in the bloodstream and binds to GLP-1 and GIP receptors in various tissues — including the pancreas, brain, gastrointestinal tract, liver, and fat tissue. The drug is slowly broken down and eliminated through normal metabolic pathways, with most of the medication cleared over several weeks.
Metabolic and Anti-Inflammatory Effects
Beyond blood sugar and weight control, tirzepatide appears to have additional metabolic and possibly anti-inflammatory effects. Studies in people with obesity and diabetes have shown improvements in markers like C-reactive protein (CRP), which is linked to systemic inflammation. These findings suggest that tirzepatide may not only regulate metabolism but also reduce low-grade chronic inflammation — a process that plays a role in conditions such as cardiovascular disease and autoimmune disorders.
By improving insulin sensitivity, reducing fat buildup in the liver, and lowering inflammatory markers, tirzepatide could indirectly benefit patients who suffer from diseases driven by inflammation — including, potentially, rheumatoid arthritis. Researchers are now studying these wider effects to understand whether tirzepatide can influence immune pathways directly.
Distinct Features Compared to Older Drugs
Tirzepatide stands out from older medications for several reasons:
- Dual receptor activation: Works through both GIP and GLP-1 receptors, producing broader metabolic effects.
- Greater weight loss: Patients often experience more significant reductions in body weight compared to traditional GLP-1 receptor agonists.
- Improved insulin sensitivity: Beyond stimulating insulin release, tirzepatide helps the body use insulin more effectively.
- Potential cardiovascular benefits: Clinical trials show improvements in lipid levels and blood pressure, which may reduce cardiovascular risk.
- Once-weekly dosing: The long-acting design makes it convenient and easy to maintain treatment adherence.
Tirzepatide is a dual incretin receptor agonist that represents an important step forward in metabolic medicine. Its ability to improve glucose control, promote weight loss, and possibly reduce inflammation makes it a strong candidate for study in diseases beyond diabetes — including rheumatoid arthritis. Although tirzepatide is not currently approved for autoimmune conditions, its unique mechanism of targeting metabolic and inflammatory pathways together provides a promising foundation for future research.
Understanding Rheumatoid Arthritis: Pathophysiology and Inflammatory Mechanisms
Rheumatoid arthritis (RA) is a long-term autoimmune disease that mainly affects the joints but can also harm other parts of the body such as the heart, lungs, and blood vessels. It develops when the body’s immune system, which normally protects against infection, begins to attack the lining of the joints. This attack causes swelling, pain, and stiffness that can lead to joint damage if not controlled. Understanding how RA develops helps explain why certain new drugs, including tirzepatide, are being studied for their possible benefits.
The Autoimmune Process in RA
In healthy joints, the inner lining called the synovium produces fluid that lubricates and nourishes the cartilage and bones. In RA, the immune system mistakenly recognizes proteins in the synovium as foreign. Immune cells such as T cells, B cells, and macrophages enter the joint space and trigger inflammation. Over time, this process thickens the synovial lining and forms a tissue called pannus, which releases enzymes that erode cartilage and bone.
This autoimmune process is driven by a combination of genetic and environmental factors. People with certain genes, such as HLA-DRB1, are more likely to develop RA, especially if exposed to environmental triggers like smoking or chronic gum infection. Once the immune system becomes activated, it stays in a constant state of alert, releasing molecules that promote inflammation throughout the body.
The Role of Cytokines in Inflammation
Inflammation in RA is largely controlled by signaling proteins called cytokines. These are small molecules that help immune cells communicate. In RA, several cytokines are produced in excess, including:
- Tumor necrosis factor-alpha (TNF-α) – a key driver of inflammation that increases joint swelling and pain.
- Interleukin-6 (IL-6) – promotes fever, fatigue, and the production of C-reactive protein (CRP), a marker of inflammation.
- Interleukin-1 beta (IL-1β) – causes cartilage breakdown and contributes to bone erosion.
These cytokines create a “vicious cycle” of inflammation. They recruit more immune cells to the joints, which in turn release more cytokines. This continuous immune activity leads to progressive joint destruction. Current biologic treatments for RA, such as anti-TNF and anti-IL-6 agents, are designed to block these cytokines and reduce inflammation.
Immune Cell Dysregulation
Several types of immune cells play specific roles in RA:
- T cells activate other immune cells and release pro-inflammatory cytokines.
- B cells produce antibodies against the body’s own proteins, including rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPAs). These antibodies can form immune complexes that further damage joint tissue.
- Macrophages and dendritic cells act as messengers that stimulate ongoing immune responses.
In RA, there is an imbalance between pro-inflammatory and regulatory immune cells. Normally, regulatory T cells help calm the immune system, but in RA their activity is reduced. The result is an immune system that stays “switched on,” even when there is no infection to fight.
The Link Between Metabolic Dysfunction and Inflammation
Recent research shows that RA is not just a joint disease — it is also a metabolic-inflammatory disorder. Many people with RA have insulin resistance, changes in fat metabolism, and higher rates of obesity. These metabolic changes can make inflammation worse.
Fat tissue, especially around the abdomen, releases substances called adipokines (such as leptin, resistin, and adiponectin). Some of these adipokines promote inflammation by activating immune cells and stimulating cytokine production. Obesity is therefore not only a risk factor for developing RA but can also make the disease more active and harder to control.
In addition, insulin resistance — a state where the body’s cells do not respond properly to insulin — can increase oxidative stress and the production of inflammatory molecules. This connection between metabolism and inflammation helps explain why drugs that improve metabolic health might also reduce inflammatory activity in RA.
Systemic Effects of Chronic Inflammation
The effects of inflammation in RA extend far beyond the joints. Chronic immune activation increases the risk of cardiovascular disease, type 2 diabetes, and fatty liver disease. Elevated levels of CRP and IL-6 contribute to the buildup of plaque in arteries, leading to heart attacks and strokes. Chronic inflammation also causes fatigue, muscle loss, and anemia. These systemic effects show that RA should be treated not only as a joint disease but as a whole-body condition.
The Inflammatory–Metabolic Loop
Scientists now describe RA as a condition driven by an inflammatory–metabolic loop. In this model, inflammation disrupts normal metabolism, and poor metabolic health further fuels inflammation. For example:
- High levels of TNF-α and IL-6 interfere with insulin signaling.
- Insulin resistance promotes more release of fatty acids and cytokines.
- These cytokines continue to activate immune cells in the synovium, worsening RA symptoms.
This loop can become self-sustaining, even when joint inflammation is partly controlled by standard treatments. Therefore, targeting both inflammation and metabolism could help break this cycle.
Implications for New Therapies
Understanding this close connection between immune dysfunction and metabolic health is guiding new research. Drugs like tirzepatide, which act on hormones involved in metabolism (GIP and GLP-1), may also influence inflammation through indirect pathways. By improving insulin sensitivity, reducing fat mass, and lowering oxidative stress, tirzepatide might modify some of the same mechanisms that drive inflammation in RA.
This is why scientists are increasingly exploring whether metabolic drugs could have a place in treating autoimmune diseases. The overlap between inflammatory and metabolic pathways provides a strong biological foundation for future research on tirzepatide and similar agents in rheumatoid arthritis.

Mechanistic Rationale: How Tirzepatide Could Influence Rheumatoid Arthritis
Tirzepatide is a new kind of medicine that acts on two hormone systems in the body — the glucose-dependent insulinotropic polypeptide (GIP) receptor and the glucagon-like peptide-1 (GLP-1) receptor. These two hormones, called incretins, help control blood sugar, appetite, and body weight. However, growing evidence suggests they may also affect the immune system and inflammation. Because rheumatoid arthritis (RA) is both an immune and metabolic disease, tirzepatide may influence several key pathways involved in RA development and progression.
Receptor Distribution and Immune System Connection
Both GIP and GLP-1 receptors are found in more than just the pancreas and digestive tract. Research shows they are also present on immune cells such as macrophages, T cells, and dendritic cells, and in tissues affected by RA, like the synovial membrane that lines the joints. This means tirzepatide can interact directly with immune cells that drive inflammation in RA.
In RA, immune cells become overactive and release chemical messengers called cytokines — such as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6) — that cause swelling, pain, and joint damage. Activation of GLP-1 and GIP receptors may reduce this inflammatory response. Studies on similar drugs have shown decreased cytokine production and lower immune cell activation when incretin receptors are stimulated. This anti-inflammatory effect could be part of how tirzepatide benefits patients with chronic inflammation.
Modulation of Macrophage and T-Cell Activity
Macrophages are immune cells that play a central role in RA. In the inflamed joint, they release enzymes and cytokines that damage tissue and bone. Tirzepatide, through GLP-1 and GIP signaling, may shift macrophages from a pro-inflammatory “M1” type to an anti-inflammatory “M2” type. This shift can calm inflammation and promote healing in the joint tissue.
Tirzepatide may also affect T cells, another key player in RA. T cells help regulate immune responses but in RA they become misdirected, attacking the body’s own tissues. GLP-1 receptor activation has been shown to promote regulatory T cells (Tregs), which help suppress harmful immune activity. This rebalancing of the immune system — reducing harmful T cells and enhancing protective ones — could help limit joint damage over time.
Reduction of Pro-Inflammatory Cytokines
Inflammation in RA is driven by a network of signaling molecules, especially TNF-α, IL-1β, and IL-6. These molecules amplify immune responses and cause pain and swelling. In animal and human studies with GLP-1 receptor agonists, activation of this pathway has been linked to a measurable reduction in these cytokines.
Tirzepatide’s dual action may strengthen this effect. GIP receptor signaling has been found to suppress the activation of nuclear factor kappa B (NF-κB), a key molecule that controls inflammation inside cells. When NF-κB is less active, cells produce fewer inflammatory cytokines. Together, the GLP-1 and GIP effects may create a combined anti-inflammatory response that targets both immune and metabolic inflammation.
Impact on Metabolic-Immune Cross Talk
Rheumatoid arthritis is not only an autoimmune disease — it is also linked to metabolic problems like obesity and insulin resistance. Fat tissue, especially around the abdomen, produces inflammatory substances called adipokines (such as leptin and resistin). These substances worsen systemic inflammation and may make RA symptoms more severe.
Tirzepatide helps reduce body weight and improves insulin sensitivity. Weight loss decreases fat mass and lowers levels of harmful adipokines, while improving the release of protective ones like adiponectin. This balance helps reduce background inflammation in the whole body. Better glucose control also reduces oxidative stress — a harmful process that contributes to joint tissue damage.
By improving metabolism, tirzepatide may therefore lower the chronic, low-grade inflammation that fuels autoimmune activity in RA. This “metabolic reset” could make existing RA treatments more effective and improve overall disease control.
Effects on Endothelial Function and Oxidative Stress
RA often comes with a higher risk of heart and blood vessel disease. Chronic inflammation damages the endothelium — the inner lining of blood vessels — leading to stiffness, poor circulation, and higher cardiovascular risk. GLP-1 receptor agonists, including those similar to tirzepatide, have shown protective effects on the endothelium. They help increase nitric oxide production, which relaxes blood vessels and improves blood flow.
Additionally, tirzepatide may reduce oxidative stress, a condition in which unstable molecules (free radicals) damage cells. Less oxidative stress means less inflammation and slower joint destruction. These vascular and oxidative benefits add another layer to tirzepatide’s potential usefulness in RA, especially for patients with metabolic or cardiovascular complications.
Current Evidence Linking Tirzepatide to Rheumatoid Arthritis Outcomes
Research directly linking tirzepatide to rheumatoid arthritis (RA) is still in its early stages. However, several lines of evidence—from laboratory studies, related clinical research, and indirect observations—suggest that tirzepatide might influence inflammation and immune activity in ways that could be relevant to RA. This section reviews what is currently known from preclinical studies, metabolic research, and comparisons with similar drugs that act on related pathways.
Evidence from Preclinical and Translational Studies
Most of the available data come from laboratory and animal studies rather than human trials. In preclinical research, tirzepatide and other drugs that act on GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotropic polypeptide) receptors have shown several effects that could theoretically benefit people with inflammatory diseases such as RA.
For example, studies in mice and cell cultures show that activation of the GLP-1 receptor can reduce the activity of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1 beta (IL-1β). These cytokines play a central role in the inflammation that damages joints in RA. GLP-1 receptor stimulation may also help shift immune cell balance by promoting anti-inflammatory macrophages (called M2 macrophages) and suppressing pro-inflammatory ones (M1 macrophages). This shift can reduce the production of damaging immune signals in joint tissues.
Tirzepatide’s dual action—stimulating both GIP and GLP-1 receptors—may provide an even stronger anti-inflammatory effect. While the GIP pathway is less studied, early data suggest that it might reduce oxidative stress and protect tissues from damage caused by long-term inflammation. In some animal models of metabolic syndrome, GIP receptor activation lowered markers of systemic inflammation and improved insulin sensitivity. Both of these effects are relevant to RA, where chronic inflammation and metabolic imbalance often coexist.
Observations from Metabolic and Inflammatory Disease Studies
Although tirzepatide has not yet been tested in large-scale clinical trials for RA, evidence from other conditions can give important clues. The drug has shown strong anti-inflammatory effects in patients with type 2 diabetes and obesity—two conditions known to increase systemic inflammation and worsen autoimmune disease outcomes.
In clinical trials for diabetes and obesity, tirzepatide consistently reduced levels of C-reactive protein (CRP), a key marker of systemic inflammation that is also used to monitor disease activity in RA. Reductions in CRP suggest that the drug may dampen overall inflammatory processes, even though these studies were not conducted in patients with autoimmune diseases.
Beyond CRP, tirzepatide also improved lipid profiles and insulin sensitivity, both of which are linked to lower inflammatory burden. Chronic insulin resistance is known to amplify immune activation, leading to higher production of inflammatory cytokines. By improving insulin sensitivity and glucose metabolism, tirzepatide may help reduce the cycle of metabolic stress that contributes to inflammation in RA.
Furthermore, weight loss itself can lower inflammation. Fat tissue releases inflammatory substances known as adipokines, including leptin and resistin, which are elevated in both obesity and RA. By promoting substantial and sustained weight loss, tirzepatide indirectly reduces these pro-inflammatory signals, potentially improving disease control for individuals with RA who are overweight or obese.
Comparisons with GLP-1 Receptor Agonists
Because tirzepatide acts partly through the GLP-1 receptor, it is useful to look at what is already known about GLP-1 receptor agonists, such as semaglutide and liraglutide, in autoimmune and inflammatory diseases. Several small clinical studies and case reports suggest that GLP-1 receptor agonists can lower inflammatory markers and improve outcomes in related conditions like psoriasis, systemic lupus erythematosus, and even experimental models of arthritis.
For example, animal studies using GLP-1 agonists showed reduced joint swelling, lower TNF-α levels, and improved histological signs of inflammation in the synovial membrane. Some observational studies in humans with diabetes found that those taking GLP-1 agonists had lower circulating inflammatory markers and fewer flare-like symptoms of joint pain compared to those on other glucose-lowering drugs. These findings support the idea that the GLP-1 pathway influences inflammation beyond its metabolic effects.
Since tirzepatide activates both GLP-1 and GIP receptors, it may amplify these benefits. However, this remains theoretical until confirmed in controlled studies specific to RA.
Indirect Clinical Observations and Emerging Data
While no completed clinical trials yet evaluate tirzepatide specifically for RA, anecdotal observations from real-world use and related metabolic studies are encouraging. Patients using tirzepatide for diabetes management have shown improvements in energy, mobility, and joint discomfort, though these outcomes were not measured as formal endpoints. Whether these effects stem from direct anti-inflammatory activity or secondary benefits such as weight loss remains unclear.
A few early research groups are beginning to explore tirzepatide’s effects in broader inflammatory contexts. Preliminary conference abstracts and preprints suggest that researchers are interested in its potential to reduce chronic low-grade inflammation, improve endothelial function, and modulate immune responses. However, results from RA-specific populations have not yet been published.
Limitations and Need for Direct Research
Despite the promising signals, the evidence base remains very limited. Most studies were not designed to test tirzepatide in RA, and findings from diabetes or obesity research cannot be automatically applied to autoimmune diseases. The immune system in RA is complex, involving not only metabolic inflammation but also autoantibody production and joint-specific immune activation. Tirzepatide may affect some but not all of these pathways.
Additionally, the duration of most tirzepatide studies has been relatively short—typically under one year. RA is a chronic, lifelong disease, and understanding how tirzepatide might influence long-term inflammation, joint health, and immune balance will require longer observation periods. The drug’s effects in combination with disease-modifying antirheumatic drugs (DMARDs) or biologics are also unknown.
Current evidence linking tirzepatide to rheumatoid arthritis outcomes is indirect but promising. Laboratory studies suggest that tirzepatide’s dual GIP and GLP-1 receptor activation can reduce inflammation and oxidative stress. Clinical data from diabetes and obesity trials show improvements in inflammatory markers such as CRP, likely related to both metabolic and immune modulation. Comparisons with GLP-1 receptor agonists further support a potential anti-inflammatory role. However, until dedicated clinical trials in RA are completed, these findings should be viewed as preliminary. The ongoing research provides a strong rationale for future studies to clarify tirzepatide’s role in immune-mediated inflammatory diseases like rheumatoid arthritis.
What Clinical Trials Are Underway or Planned?
Tirzepatide is a relatively new medication, first approved for type 2 diabetes and later for obesity management. Because of its strong effects on weight, blood sugar, and inflammation, researchers are now beginning to study whether it could also help people with autoimmune diseases such as rheumatoid arthritis (RA). While there are currently no large clinical trials focused solely on tirzepatide for RA, several ongoing studies and planned investigations are exploring its effects on inflammation, immune regulation, and related metabolic disorders. Understanding these studies helps explain where the research is heading and what scientists hope to discover in the next few years.
Current Registered Clinical Trials
Most registered tirzepatide trials are focused on metabolic conditions like type 2 diabetes, obesity, and metabolic-associated fatty liver disease (MAFLD). However, many of these studies include inflammatory biomarkers—such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and interleukin-6 (IL-6)—as secondary outcomes. These markers are also used to monitor inflammation in rheumatoid arthritis. As a result, these metabolic trials may provide valuable indirect information about tirzepatide’s possible role in lowering systemic inflammation that contributes to RA symptoms.
For example, large phase 3 trials like SURPASS-1 through SURPASS-5 (in type 2 diabetes) and SURMOUNT-1 (in obesity) have already shown that tirzepatide reduces inflammatory markers and improves lipid profiles. In many participants, CRP levels dropped significantly after several months of treatment, suggesting that tirzepatide’s anti-inflammatory effects may extend beyond blood sugar control. While these studies were not designed for arthritis patients, they offer early evidence that tirzepatide could help reduce chronic low-grade inflammation—an important feature of rheumatoid arthritis.
Some smaller studies are now being planned to look directly at autoimmune or inflammatory diseases. For instance, exploratory clinical trials are being developed to assess tirzepatide’s effect on:
- Inflammatory joint pain and stiffness in overweight or insulin-resistant individuals.
- Systemic inflammatory markers in patients with metabolic syndrome who also show early signs of autoimmune inflammation.
- Cardiometabolic inflammation, which overlaps with conditions like RA that involve both immune and metabolic dysregulation.
Although these trials are in the early stages of design or recruitment, they represent the first step toward applying tirzepatide’s dual GIP/GLP-1 mechanism to immune-driven conditions.
Key Clinical Endpoints and Measures
To understand whether tirzepatide truly benefits RA, future clinical trials will likely measure both inflammatory and clinical outcomes. These may include:
- CRP and ESR levels – common blood tests for inflammation.
- Disease Activity Score in 28 joints (DAS28) – a standard measure of joint swelling and tenderness used in RA trials.
- American College of Rheumatology (ACR) response criteria – which evaluate how well patients improve based on joint counts, pain scores, and lab results.
- Quality-of-life assessments, such as fatigue, pain perception, and physical function questionnaires.
- Imaging endpoints using ultrasound or MRI to track joint inflammation and damage.
Researchers may also explore molecular biomarkers such as TNF-α, IL-1β, and IL-6, which are key cytokines in RA inflammation. If tirzepatide can lower these markers, it would support its potential role as an immune-modulating therapy.
Preliminary Findings and Early Insights
Although no phase 3 trials yet target rheumatoid arthritis, early results from metabolic studies are encouraging. Participants receiving tirzepatide have shown:
- Reduced systemic inflammation as measured by CRP and IL-6.
- Improved insulin sensitivity, which may lower immune cell activation.
- Weight reduction that correlates with less joint pain and stiffness in those with arthritis risk factors.
- Better cardiovascular and endothelial function, which could indirectly improve RA outcomes, since patients with RA have high cardiovascular risk.
In some post-hoc analyses, researchers noted that tirzepatide improved markers of liver inflammation and adipose tissue inflammation. These findings suggest that tirzepatide’s anti-inflammatory actions may be widespread, not limited to metabolic organs. This cross-system benefit is particularly important for RA, where inflammation occurs throughout the body, not only in joints.
Research Gaps and Challenges
Despite these promising signs, several important gaps remain. First, there is still no randomized controlled trial (RCT) designed specifically to evaluate tirzepatide in people diagnosed with RA. Without direct evidence from such trials, doctors cannot yet recommend tirzepatide for arthritis treatment.
Second, most available data come from populations with diabetes or obesity, not autoimmune disease. These groups may respond differently to metabolic drugs because their immune systems behave in distinct ways. Therefore, more research is needed to understand how tirzepatide’s immune effects translate to autoimmune conditions like RA.
Third, dosing and duration for anti-inflammatory benefit are not yet established. It is unclear whether standard tirzepatide doses (used for diabetes or weight loss) would provide adequate immune modulation, or if different dosing schedules are needed for RA patients.
Lastly, future research must evaluate safety and interactions with common RA medications, such as methotrexate or biologic drugs. Since many RA patients already take multiple immunomodulatory agents, understanding how tirzepatide fits into combination therapy is essential.
The Road Ahead
The next few years will likely bring new clinical studies exploring tirzepatide’s effects on inflammation, joint pain, and autoimmune disease progression. Some early pilot trials may begin by recruiting overweight RA patients who have metabolic comorbidities such as insulin resistance or dyslipidemia. These studies will help determine whether tirzepatide’s benefits on metabolism can translate into measurable improvements in disease activity.
If results are positive, larger phase 2 and phase 3 trials could follow—potentially making tirzepatide one of the first metabolic drugs to be tested systematically for rheumatoid arthritis. Such research would also pave the way for studying other incretin-based therapies in autoimmune diseases.
Tirzepatide is entering a new research stage that bridges metabolism and immunity. While the current evidence is mostly indirect, the scientific interest is strong, and well-designed clinical trials are on the horizon. These studies will answer whether tirzepatide can go beyond glucose control to become part of the future of rheumatoid arthritis therapy.
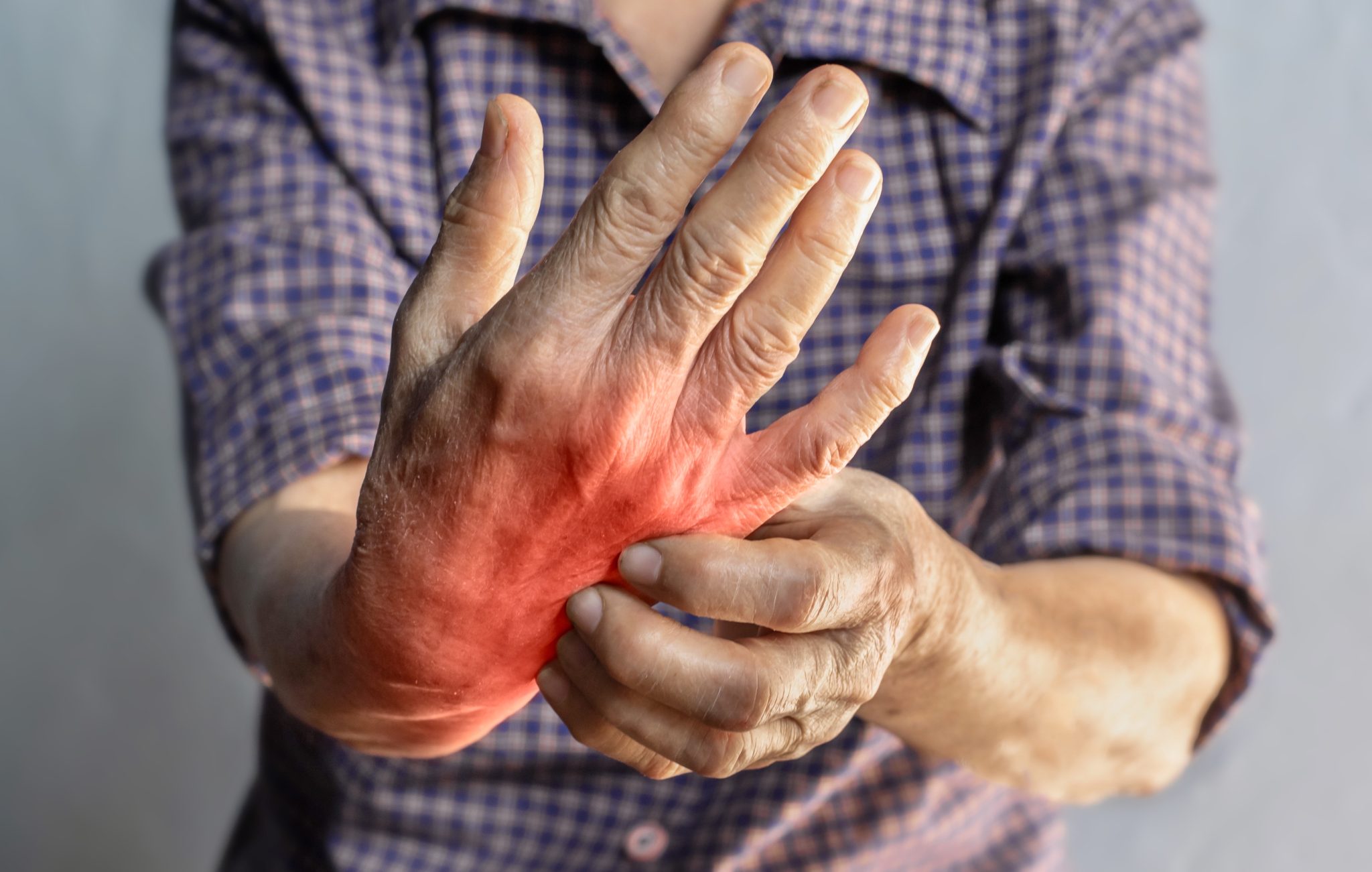
Potential Benefits of Tirzepatide in Rheumatoid Arthritis
Tirzepatide may offer several benefits for people living with rheumatoid arthritis (RA), even though it is not yet an approved treatment for this condition. Its effects on metabolism, inflammation, and body weight may work together to improve disease control, reduce symptoms, and protect long-term health. While more studies are needed, scientists have identified several possible ways tirzepatide could help patients with RA.
Reduction in Systemic Inflammation Through Metabolic Control
Rheumatoid arthritis is a disease driven by chronic inflammation. This inflammation comes not only from the immune system but also from metabolic imbalance. High blood sugar, insulin resistance, and excess body fat can all increase inflammatory chemicals such as C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6).
Tirzepatide works by activating two hormone pathways: the glucose-dependent insulinotropic polypeptide (GIP) receptor and the glucagon-like peptide-1 (GLP-1) receptor. These hormones help regulate blood sugar, improve insulin sensitivity, and reduce body fat. When metabolism improves, inflammatory stress in the body can decline.
Animal and early human studies suggest that GLP-1 receptor activation may reduce the activity of immune cells that produce inflammatory cytokines. It may also shift immune balance toward anti-inflammatory responses. If tirzepatide can lower these inflammation signals in people with RA, it might reduce the frequency or severity of flare-ups.
Because systemic inflammation is a key driver of joint damage in RA, any reduction could have a meaningful effect on disease progression and quality of life.
Weight Loss Benefits in Overweight RA Populations
Obesity is common among people with rheumatoid arthritis and is known to worsen symptoms and reduce the effectiveness of treatment. Excess weight increases the mechanical load on joints and promotes the release of pro-inflammatory molecules from fat tissue, called adipokines.
Tirzepatide has been shown in clinical trials for diabetes and obesity to produce significant and sustained weight loss—often 15% to 20% of body weight over time. This reduction could help relieve joint stress, improve mobility, and lower inflammation throughout the body.
Weight loss may also improve response to disease-modifying antirheumatic drugs (DMARDs) and biologics. In studies of other weight-loss medications, patients with lower body fat often had lower disease activity and reported less pain and stiffness. If tirzepatide can produce similar benefits, it could help complement existing RA treatments.
Cardiovascular Protection and Metabolic Improvements
People with rheumatoid arthritis face a higher risk of cardiovascular disease, including heart attack and stroke. This risk comes from chronic inflammation, but also from metabolic factors like insulin resistance, high cholesterol, and obesity.
Tirzepatide has been shown to improve several of these risk factors. It can lower blood glucose, reduce low-density lipoprotein (LDL) cholesterol, increase high-density lipoprotein (HDL) cholesterol, and lower blood pressure. These effects can lessen the overall cardiovascular burden that often complicates long-term RA management.
Reducing systemic inflammation may also protect blood vessels from the damage that contributes to atherosclerosis. By improving both metabolic and inflammatory health, tirzepatide could play a dual protective role, addressing two major drivers of heart disease in RA patients.
Improvements in Energy, Fatigue, and Physical Function
Fatigue is one of the most disabling symptoms of rheumatoid arthritis. It often persists even when joint inflammation is well controlled. Research shows that fatigue can be linked to high blood sugar, poor sleep, and chronic inflammation—all of which tirzepatide may help improve.
By stabilizing blood sugar and promoting weight loss, tirzepatide can increase energy levels and support better sleep quality. Patients in diabetes studies often reported feeling more energetic and less fatigued after several months of treatment.
Better energy can lead to more physical activity, which in turn strengthens muscles, reduces stiffness, and supports joint health. In the context of RA, this cycle of improvement could contribute to a better overall sense of well-being.
Integrating Metabolic and Inflammatory Therapeutic Effects
Rheumatoid arthritis is both an immune disease and a metabolic condition. Metabolic disturbances can amplify immune activation, and inflammation can worsen insulin resistance. Tirzepatide targets this overlap between metabolism and immunity.
Its dual hormone activity helps rebalance these systems, offering a “whole-body” approach to managing chronic inflammation. This makes tirzepatide especially interesting for patients who have both RA and metabolic conditions like type 2 diabetes or obesity.
In theory, improving metabolic health could enhance the effect of standard RA medications. For example, lower systemic inflammation might make biologic therapies more effective or reduce the dose needed to control symptoms. While this synergy has not yet been proven in clinical trials, it represents a promising direction for future research.
Tirzepatide may benefit people with rheumatoid arthritis by improving metabolic control, reducing inflammation, promoting weight loss, and protecting cardiovascular health. It may also help with fatigue and physical function through its effects on energy and body composition.
Although these benefits are based on early data and indirect evidence, the potential overlap between metabolic and inflammatory pathways provides a strong scientific reason to continue studying tirzepatide in RA. If future trials confirm its effects, tirzepatide could become a valuable addition to comprehensive care for patients living with this complex disease.
Safety, Tolerability, and Considerations in RA Populations
Tirzepatide has shown strong effects in people with type 2 diabetes and obesity, but its use in rheumatoid arthritis (RA) is still experimental. While it may offer metabolic and anti-inflammatory benefits, it is important to understand how safe it might be for people living with RA, who often take multiple medications and have unique health challenges. This section reviews what is currently known about tirzepatide’s safety, common side effects, drug interactions, and considerations for use in patients with autoimmune diseases like RA.
Common Side Effects and Tolerability
In clinical trials for diabetes and obesity, the most common side effects of tirzepatide have been related to the digestive system. Many patients experience nausea, vomiting, diarrhea, constipation, or abdominal pain during the first few weeks of treatment. These effects usually occur because tirzepatide slows down stomach emptying, a normal part of its mechanism.
For most people, these symptoms are mild to moderate and tend to improve as the body adjusts to the medication. Starting at a low dose and increasing gradually helps reduce discomfort. However, people with RA who already have stomach issues from medications like nonsteroidal anti-inflammatory drugs (NSAIDs) or corticosteroids might experience greater irritation or digestive distress.
Fatigue and loss of appetite are also possible. While appetite loss contributes to weight reduction, sudden or excessive weight loss could lead to muscle loss or weakness, which may worsen fatigue or affect physical strength in RA patients. Therefore, it is important to monitor weight changes carefully and maintain proper nutrition.
Drug Interactions and Combination Therapy
Rheumatoid arthritis patients often take disease-modifying antirheumatic drugs (DMARDs) such as methotrexate, hydroxychloroquine, or biologic agents like adalimumab and etanercept. At this time, there is no direct evidence of harmful interactions between tirzepatide and these therapies. However, because tirzepatide affects digestion and the absorption of oral drugs, it could theoretically change how some RA medications are absorbed. This is especially relevant for oral methotrexate or corticosteroids.
Clinicians may need to space doses apart or monitor blood levels and side effects more closely when tirzepatide is introduced. People taking insulin or other glucose-lowering drugs must also be careful, as tirzepatide can enhance insulin sensitivity, increasing the risk of hypoglycemia (low blood sugar). While hypoglycemia is rare in people without diabetes, it can occur if combined with other drugs that lower glucose or if food intake is reduced significantly.
Because tirzepatide is a relatively new medication, long-term drug–drug interaction data are still being collected. Physicians treating RA patients considering tirzepatide will need to evaluate the full medication list, including supplements and over-the-counter drugs, before prescribing it.
Considerations for Patients with Comorbidities
RA patients often have several other health conditions, such as heart disease, high blood pressure, obesity, and type 2 diabetes. In these cases, tirzepatide might offer extra benefits because it improves blood sugar control, reduces weight, and lowers inflammation, all of which can improve cardiovascular health.
However, patients with advanced heart failure or those on multiple cardiac medications should be monitored carefully. Tirzepatide can cause mild increases in heart rate, and dehydration from vomiting or diarrhea can worsen heart or kidney function.
Patients with kidney or liver disease should also use tirzepatide cautiously. While clinical trials have not shown major safety issues, limited data exist for people with moderate or severe organ impairment. Regular kidney and liver tests may be recommended to ensure safe use.
Contraindications and Specific Risk Profiles
Tirzepatide should not be used by anyone with a personal or family history of medullary thyroid carcinoma (MTC) or multiple endocrine neoplasia syndrome type 2 (MEN2). These warnings come from animal studies showing an increased risk of thyroid C-cell tumors. Although this risk has not been proven in humans, it remains a strong precaution.
Similarly, patients with a history of pancreatitis (inflammation of the pancreas) should avoid or use tirzepatide with close medical supervision, since GLP-1–related drugs have occasionally been linked to this rare but serious condition.
For women of childbearing potential, safety during pregnancy and breastfeeding has not been established. Tirzepatide is generally avoided during pregnancy because rapid weight loss and hormonal changes could affect fetal development.
Post-Marketing Safety and Real-World Experience
Tirzepatide has been on the market for a shorter time than older GLP-1 drugs like semaglutide or liraglutide, so long-term safety data are still being gathered. Real-world studies from people using it for diabetes or weight management have reported similar side effects to clinical trials, with gastrointestinal symptoms being the most frequent.
So far, there have been no major safety signals indicating severe risks beyond those already known. However, because RA patients may take immune-modulating or liver-metabolized drugs, more specific post-marketing data are needed for this group. Ongoing pharmacovigilance programs and real-world registries will provide better insights into its safety in autoimmune populations.
Overall, tirzepatide appears to have a manageable safety profile when used in people with diabetes and obesity, and these findings offer cautious optimism for its use in rheumatoid arthritis research. However, because RA patients often face complex health issues and medication combinations, individualized care and close medical supervision are essential.
Until clinical trials in RA are completed, tirzepatide should not be used as a routine therapy for inflammation but may become a promising option in the future if shown to be both effective and safe for this population.
Challenges and Research Gaps
Although tirzepatide shows early promise for inflammatory diseases like rheumatoid arthritis (RA), there are still many challenges and unanswered questions before it can be considered a safe and effective treatment option. Much of the current information comes from indirect evidence—mainly from its use in diabetes and obesity. To truly understand its impact on RA, researchers must explore several important scientific and clinical gaps.
Lack of Disease-Specific Clinical Trials
The biggest challenge is that no large, well-designed clinical trials have yet tested tirzepatide directly in people with rheumatoid arthritis. Most of the data we have comes from studies on patients with type 2 diabetes or obesity, where researchers noticed improvements in inflammation markers such as C-reactive protein (CRP). While these findings are interesting, they cannot confirm whether tirzepatide directly improves RA symptoms such as joint pain, swelling, and fatigue.
Rheumatoid arthritis is a complex autoimmune disease. Its treatment effects need to be measured with specific clinical tools, like the Disease Activity Score (DAS28), erythrocyte sedimentation rate (ESR), and American College of Rheumatology (ACR) response criteria. Without these disease-specific trials, it is impossible to know whether tirzepatide helps reduce inflammation in the joints or prevents long-term damage.
To close this gap, future studies need to include patients with confirmed RA diagnoses and compare tirzepatide with standard treatments like methotrexate or biologic disease-modifying antirheumatic drugs (DMARDs). Placebo-controlled trials will also be necessary to identify whether benefits are truly due to tirzepatide’s mechanism or secondary to weight loss and metabolic improvements.
Biological Complexity of Dual Incretin Signaling
Tirzepatide acts on two hormonal pathways: the GLP-1 receptor and the GIP receptor. Both are known to influence not only glucose metabolism but also the immune system. However, scientists do not yet fully understand how these pathways interact in autoimmune conditions like RA.
For example, GLP-1 receptor activation can reduce inflammatory cytokines such as TNF-α and IL-6, which play major roles in RA. On the other hand, the function of GIP signaling in immune regulation is less clear. Some studies suggest that GIP may increase bone formation and reduce inflammation, but others indicate that it could have neutral or even opposite effects under certain conditions. This mixed evidence makes it difficult to predict how dual agonism will affect autoimmune inflammation in humans.
The complexity increases when considering that RA patients often have different metabolic states—some are overweight, while others have rheumatoid cachexia (muscle loss with inflammation). Tirzepatide’s effects may vary widely depending on each patient’s metabolism, immune profile, and medication history. Understanding these biological variations will be key to determining who might benefit most from tirzepatide therapy.
Translational Limitations and Knowledge Gaps
Most of what we know about tirzepatide and inflammation comes from preclinical animal studies or indirect human observations. Animal models can show useful insights into immune pathways, but they do not always predict real-world results in people. For instance, the metabolism and immune system of rodents differ from humans in key ways. A drug that works well in mice may not have the same safety or effectiveness in patients with complex diseases like RA.
In addition, current studies focus mainly on metabolic or cardiovascular outcomes, not on autoimmunity. Researchers still do not know if tirzepatide reaches the inflamed synovial tissue in RA or if its anti-inflammatory effects are strong enough to change disease progression. There are also unanswered questions about its long-term impact on bone health, joint protection, and immune tolerance.
Need for Biomarkers and Patient Selection
To study tirzepatide effectively in RA, researchers need reliable biomarkers—biological signals that can measure inflammation or treatment response. Markers like CRP and ESR are useful but not specific enough to detect early immune changes. More precise biomarkers, such as cytokine profiles or metabolic signatures, could help identify which patients respond best to tirzepatide.
Personalized medicine approaches are becoming more important in rheumatology. It is likely that tirzepatide will not work the same way for all patients. Those with obesity or metabolic syndrome may benefit more than those with normal weight or severe, treatment-resistant RA. Developing a patient selection strategy will improve the design of future clinical trials and reduce the risk of misleading results.
Ethical and Logistical Challenges in Repurposing Metabolic Drugs
Using a metabolic drug like tirzepatide for an autoimmune disease raises ethical and practical issues. Because tirzepatide is still relatively new, its long-term safety profile is not fully known outside diabetes and obesity populations. RA patients often take multiple medications, including corticosteroids and immunosuppressants, which could increase the risk of side effects or drug interactions.
Designing safe and ethical clinical trials will require careful screening and close monitoring of participants. It will also demand collaboration between endocrinologists, rheumatologists, and pharmacologists to ensure that metabolic effects are properly managed during testing. Furthermore, regulatory agencies will need strong evidence of both safety and efficacy before approving tirzepatide for use in RA.
Tirzepatide represents an exciting frontier in the overlap between metabolism and immune regulation. However, before it can be considered a real option for rheumatoid arthritis, researchers must address the many scientific, ethical, and practical challenges described above. Future studies that focus on RA-specific outcomes, immune pathways, and patient selection will be essential to unlocking the drug’s true potential in autoimmune disease management.

Future Prospects and Directions
Tirzepatide represents a new and interesting approach to managing chronic diseases that involve both metabolism and inflammation. While it is currently approved for type 2 diabetes and obesity, its dual action on GIP and GLP-1 receptors has drawn attention from researchers studying autoimmune and inflammatory conditions such as rheumatoid arthritis (RA). In the coming years, several areas of investigation may shape how tirzepatide fits into the treatment landscape for RA. These include the integration of metabolic therapies in autoimmune disease management, the use of combination treatments, the identification of new biomarkers, personalized approaches to therapy, and stronger collaboration between different medical specialties.
Integration of Metabolic Therapies in Autoimmune Disease Management
Traditional treatments for RA focus mainly on suppressing the immune system to reduce inflammation and prevent joint damage. These include disease-modifying antirheumatic drugs (DMARDs), biologics, and targeted synthetic agents. However, many people with RA also live with metabolic conditions like obesity, insulin resistance, and cardiovascular disease. These comorbidities can worsen inflammation and make RA harder to control.
Tirzepatide offers an opportunity to target these overlapping pathways. By improving insulin sensitivity, promoting weight loss, and reducing systemic inflammation, it may indirectly help lower disease activity in RA. In the future, metabolic agents such as tirzepatide might be used alongside traditional immune-modulating treatments to create a more holistic approach — one that treats both inflammation and the metabolic disturbances that fuel it.
This approach reflects a broader shift in medicine: recognizing that chronic inflammation is not only an immune problem but also a metabolic one. If future studies confirm that metabolic therapies can reduce inflammatory burden in RA, treatment strategies could expand beyond immune suppression to include drugs that optimize metabolism and energy balance.
Potential for Combination Therapy with DMARDs or Biologics
It is unlikely that tirzepatide alone would replace conventional RA drugs, but it might complement them. Combination therapy could provide multiple benefits — for example, reducing inflammation through both metabolic and immune mechanisms while also improving weight and cardiovascular health.
For patients who have partial responses to biologics or DMARDs, adding tirzepatide could theoretically enhance outcomes. This would be especially valuable in overweight or insulin-resistant individuals, who tend to have higher levels of inflammation and poorer responses to standard treatments.
Future trials may test tirzepatide as an add-on therapy, assessing its ability to reduce markers such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), as well as its impact on clinical scores like DAS28. Safety and drug–drug interaction data will also be crucial before such combinations can be recommended in practice.
Emerging Biomarkers to Monitor Metabolic-Immune Interactions
To understand how tirzepatide affects RA, scientists will need to track biomarkers that connect metabolism and immunity. Traditional inflammation markers like CRP and ESR may not tell the full story. Instead, future studies could examine specific cytokines, adipokines (such as leptin and adiponectin), and metabolic markers like fasting insulin, HOMA-IR, or lipid particle profiles.
Changes in these markers may reveal how metabolic therapies influence immune pathways at the cellular level. Identifying reliable biomarkers will also help predict which patients are most likely to respond to tirzepatide-based therapy. This could make clinical trials more efficient and help tailor treatment plans for individual patients.
Personalized Medicine Approaches
As research advances, it is becoming clear that RA is not a single disease but a spectrum of related disorders with different biological drivers. Some patients have more immune-driven inflammation, while others may have strong metabolic or vascular components.
Tirzepatide might be most effective in specific subgroups — for example, patients with obesity, metabolic syndrome, or type 2 diabetes who also have RA. Personalized medicine approaches could use genetic, metabolic, and immune profiling to identify these individuals. This would allow clinicians to target therapy more precisely, improving effectiveness while reducing unnecessary treatment in those unlikely to benefit.
Multi-Disciplinary Collaboration
Finally, successful integration of tirzepatide into RA care will require cooperation between multiple medical specialties. Rheumatologists, endocrinologists, cardiologists, and clinical pharmacologists will need to work together to evaluate outcomes across different systems — joints, metabolism, and the cardiovascular system. Such collaboration can improve patient safety and ensure that clinical trials address both the immune and metabolic dimensions of RA.
In addition, this cross-disciplinary work may open new fields of research into other inflammatory diseases, such as lupus, psoriatic arthritis, and inflammatory bowel disease, where metabolic factors also play a role. Tirzepatide could serve as a model for how metabolic drugs might be repurposed to treat complex immune disorders.
The next decade is likely to bring a deeper understanding of how metabolism and immunity interact. If ongoing research confirms tirzepatide’s ability to reduce inflammation while improving metabolic health, it could mark the beginning of a new era in rheumatoid arthritis management. Instead of focusing only on immune suppression, future treatments might target the broader network of biological systems that contribute to chronic inflammation.
Tirzepatide’s potential in RA lies not only in its dual receptor activity but also in the new therapeutic philosophy it represents — one that bridges metabolism, immunity, and systemic health. Continued clinical studies, biomarker research, and cross-specialty collaboration will be essential to unlock its full promise for people living with rheumatoid arthritis.
Conclusion
Tirzepatide represents a new class of medication that bridges the gap between metabolic and inflammatory diseases. Although it was developed to treat type 2 diabetes and obesity, its unique dual action on glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors has opened new doors for research into autoimmune and chronic inflammatory disorders such as rheumatoid arthritis (RA). This interest is not random—scientists now understand that inflammation and metabolism are deeply connected. When the body’s metabolic balance is disturbed, it can increase inflammation, and when inflammation is long-term, it can worsen metabolic health. RA sits at this intersection, where immune cells, cytokines, and metabolic pathways interact continuously.
At present, RA is treated mainly with disease-modifying antirheumatic drugs (DMARDs), biologics, and Janus kinase (JAK) inhibitors. These medications aim to control the immune system and reduce joint inflammation. While effective, many patients still struggle with fatigue, metabolic syndrome, obesity, and insulin resistance. These problems can worsen inflammation, make RA harder to control, and raise the risk of heart disease. Because tirzepatide improves both glucose and fat metabolism while also reducing inflammatory signals in other diseases, it is reasonable to explore whether it could benefit people with RA.
Mechanistically, tirzepatide could help RA patients in several ways. Its action on GLP-1 and GIP receptors can lower pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β. It may also change how macrophages behave, promoting anti-inflammatory types rather than those that sustain inflammation. Animal and laboratory studies suggest that incretin-based drugs can reduce oxidative stress and improve endothelial function, which are important for protecting blood vessels from chronic inflammation. These actions could not only calm inflammation in joints but also improve the cardiovascular health of people living with RA—a group known to have a higher risk of heart disease.
The metabolic benefits of tirzepatide are equally important. Many RA patients struggle with weight gain from corticosteroid use or limited mobility. Extra body fat, especially visceral fat, produces inflammatory molecules called adipokines, which can worsen joint inflammation and pain. By promoting significant weight loss and improving insulin sensitivity, tirzepatide could help reduce these harmful signals. Better metabolic control may therefore create a more favorable immune balance, lowering the intensity of systemic inflammation. This holistic improvement—affecting both metabolism and immunity—is what makes tirzepatide an exciting potential tool in RA management.
However, it is important to note that evidence is still limited. So far, no large-scale clinical trial has specifically studied tirzepatide in patients with RA. Most of the current understanding comes from indirect observations, preclinical studies, or research on GLP-1 receptor agonists in other inflammatory conditions. While some early data are encouraging, the scientific community needs randomized controlled trials that directly measure RA outcomes—such as disease activity scores, joint pain, and inflammatory markers—in patients using tirzepatide. Until such data are available, it would be premature to conclude that tirzepatide can serve as a treatment for RA.
Safety and tolerability are other essential considerations. Tirzepatide’s common side effects—such as nausea, vomiting, and diarrhea—are generally mild but may be challenging for patients who already take multiple medications for RA. The interaction between tirzepatide and immunosuppressive drugs has not been fully studied. Additionally, careful monitoring is needed for patients with a history of pancreatitis or thyroid disorders. Long-term studies are also required to ensure that the drug remains safe when used for chronic inflammatory conditions beyond diabetes or obesity.
Looking ahead, the future of tirzepatide in rheumatology will depend on collaboration between endocrinologists and rheumatologists. This partnership could help design trials that examine how metabolic treatments affect immune-mediated diseases. It may also lead to more personalized treatment plans that address both metabolic and inflammatory factors in each patient. The concept of using a single medication to improve metabolic health and immune regulation is a powerful one. It could change how chronic diseases like RA are treated—moving from symptom control to overall health restoration.
In summary, tirzepatide’s dual incretin mechanism offers more than blood sugar control. It represents a promising approach to modulating the immune-metabolic axis that drives inflammation in rheumatoid arthritis. The early scientific evidence supports the idea that improving metabolism can reduce inflammation and possibly improve joint health and quality of life. Yet, much remains to be proven. Rigorous clinical studies are needed to confirm the safety, efficacy, and long-term benefits of tirzepatide in RA populations. If future trials show positive outcomes, tirzepatide could become part of a new generation of therapies that treat autoimmune diseases not just by suppressing the immune system, but by restoring balance to the body’s interconnected metabolic and inflammatory pathways.
Research Citations
Kellner, D. A., Dente, E., Tran, V., Welsh, T., Tran, V., Saha, A., Baker, J. F., Elashoff, D. A., & Ranganath, V. K. (2025). Effect of glucagon-like peptide-1 receptor agonists on patients with rheumatoid arthritis. ACR Open Rheumatology, 7(9), e70103.
Dente, E., Kellner, D., Tran, V., Welsh, T., Tran, V., Saha, A., Brook, J., Elashoff, D., & Ranganath, V. (2024, November 18). Effects of anti-obesity medications in RA patients [Conference abstract]. ACR Convergence 2024, Arthritis & Rheumatology (Suppl 9).
Loizidis, G., & Summer, R. (2025, October 26). GLP-1 receptor agonists reduce mortality and cardiovascular events in patients with rheumatoid arthritis [Conference abstract]. ACR Convergence 2025, Arthritis & Rheumatology (Suppl 9).
McCormick, N., Zhang, J., Holladay, E., Xie, F., & Curtis, J. (2025). GLP-1 receptor agonists to facilitate weight loss and improve disease activity, pain and function in patients with rheumatic and musculoskeletal disease: Real-world evidence from the RISE registry [Conference abstract]. ACR Convergence 2025, Arthritis & Rheumatology (Suppl 9).
Masson, W., Lobo, M., Nogueira, J. P., Barbagelata, L., Touzas, P., & Frías, J. P. (2025). Anti-inflammatory effects of tirzepatide: A systematic review and meta-analysis. Reviews in Endocrine and Metabolic Disorders. Advance online publication.
Taktaz, F., Maroufi, A., Borji, M., & Faghihi, M. A. (2024). Bridging the gap between GLP-1 receptor agonists and cardiovascular outcomes: Evidence for the role of tirzepatide. Cardiovascular Diabetology, 23, 319.
Sattar, N., et al. (2024). Inflammatory biomarkers in people receiving tirzepatide in SURMOUNT-1 and SURMOUNT-2 [Conference abstract]. Circulation (AHA Scientific Sessions Supplement).
Min, T., Bain, S. C., & Shankar, S. S. (2020). The role of tirzepatide, dual GIP and GLP-1 receptor agonist, in the management of type 2 diabetes: The SURPASS clinical program. Diabetes Therapy, 11(3), 835–846.
Nauck, M. A., & Meier, J. J. (2022). Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes: A review. The Lancet Diabetes & Endocrinology, 10(6), 355–371.
Alharbi, S. H., & Almutairi, M. M. (2024). Anti-inflammatory role of glucagon-like peptide-1 receptor agonists: Implications for chronic inflammatory diseases. International Journal of Molecular Sciences, 25(2), 1234.
Questions and Answers: Tirzepatide for Rheumatoid Arthritis
Tirzepatide is a dual GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) receptor agonist. It was developed primarily for type 2 diabetes and obesity: by stimulating insulin release and reducing glucagon, plus slowing gastric emptying and reducing appetite, it leads to lower blood glucose and weight loss. In the rheumatoid arthritis (RA) context, the interest is partly because weight, metabolic health, and inflammation are all interconnected.
There are several theoretical or emerging reasons:
- Obesity and excess adiposity worsen RA disease activity, pain, and treatment response.
- Weight loss has been shown to reduce disease activity, fatigue, and joint symptoms in RA and other inflammatory conditions.
- Some GLP-1 receptor agonists (a related class) have shown anti-inflammatory effects, reduced CRP (C-reactive protein), and improved cardiovascular risk factors in RA patients.
Thus, while not proven yet for RA, tirzepatide has mechanistic plausibility for benefits beyond weight loss.
Direct evidence is limited.
- Some real-world studies of GLP-1 receptor agonists in RA patients found decreases in RA disease activity and pain.
- Reports from people with RA taking anti-obesity medications (including tirzepatide) noted improvements in inflammatory markers and pain.
- No large randomized controlled trials have yet been published specifically for tirzepatide in RA.
Therefore, its use in RA remains exploratory.
Possible benefits include:
- Weight and adiposity reduction, leading to less joint load and improved mobility.
- Better metabolic and cardiovascular health, common issues in RA.
- Possible anti-inflammatory effects through reduced adipose-derived cytokines and improved insulin sensitivity.
- Improvements in pain, fatigue, and quality of life if inflammation and comorbidities improve.
- Common side effects include nausea, vomiting, and constipation.
- RA patients often take immunosuppressants, so interactions and immune effects must be considered.
- Evidence for RA benefit is still very limited—use would be off-label.
- Weight loss must be monitored to ensure it is safe and not due to disease activity.
- Costs and access may also be barriers.
Preliminary trials exist for tirzepatide in psoriatic arthritis (a related inflammatory arthritis), especially in overweight or obese participants.
However, no large randomized trials have yet evaluated tirzepatide’s efficacy specifically for RA. Research is still in early stages.
- It may reduce adipose tissue inflammation, which contributes to RA disease activity.
- GLP-1 agonism has shown anti-inflammatory effects in animal models, reducing synovial inflammation and cytokines.
- It can improve insulin resistance and metabolic dysfunction, lowering systemic inflammation.
- It may also improve cardiovascular inflammation, an important comorbidity in RA.
No. Tirzepatide should not replace disease-modifying antirheumatic drugs or biologics. RA is an autoimmune disease requiring targeted immunotherapy. Tirzepatide may eventually serve as an adjunct for weight and metabolic management but not as a primary RA treatment.
- Weight, BMI, and waist circumference.
- RA disease activity scores and inflammatory markers.
- Blood glucose, HbA1c, blood pressure, and lipids.
- Cardiovascular health and side effects.
- Nutritional status and unintended weight loss.
- Interactions with RA medications.
- Liver and kidney function.
- Overall quality of life, fatigue, and pain levels.
- Does tirzepatide directly reduce RA inflammation independent of weight loss?
- What is the ideal dosing and duration for RA patients?
- Which patient subgroups would benefit most?
- What are the long-term safety outcomes in those on immunosuppressants?
- How does it interact with standard RA drugs?
- Is it cost-effective for RA care?
- How much of its benefit comes from weight loss vs. anti-inflammatory mechanisms?
These questions must be answered before tirzepatide can be routinely used in RA management.

