Table of Contents
Introduction
Tirzepatide is a new type of medicine used to treat type 2 diabetes. It is also being used more often for weight loss in people who are overweight or obese. Tirzepatide has gained attention because of how well it helps lower blood sugar and reduce body weight. It works in a different way than many older diabetes medicines, and early studies have shown strong results. Because of this, doctors are starting to prescribe it more frequently. However, as more people begin using tirzepatide, questions are being asked about its possible side effects. One of the most serious concerns is whether tirzepatide might cause kidney damage.
The kidneys are very important organs that filter waste from the blood and remove extra fluids from the body. People with diabetes already have a higher risk of kidney problems. This is because high blood sugar over time can damage the small blood vessels in the kidneys. When a person with diabetes takes a new medicine, especially one that works in new ways, doctors and patients want to be sure that the medicine is safe for the kidneys. This is especially true for those who already have some level of kidney disease or who are taking other medications that affect kidney function.
Tirzepatide belongs to a group of medicines called GLP-1 receptor agonists, but it also targets another hormone called GIP. Because it works on two hormone pathways instead of one, it is called a dual agonist. This dual action helps people feel full, eat less, and lose weight. It also helps control blood sugar by helping the body make more insulin and reducing how much sugar the liver makes. These effects are helpful for people with type 2 diabetes and obesity, but they also come with side effects. Some of the most common side effects include nausea, vomiting, and diarrhea. These side effects can lead to dehydration, which means the body loses too much fluid. When the body is dehydrated, the kidneys may not work as well. In some cases, this can lead to acute kidney injury, also known as AKI.
Concerns about kidney problems from tirzepatide mostly come from this risk of dehydration. When people take medicines that cause vomiting or diarrhea, they need to be careful to drink enough fluids. If they do not, the kidneys can become stressed and may not filter the blood properly. This can be especially dangerous for older adults, people who already have kidney disease, or those who take water pills or other drugs that affect blood pressure or fluid balance.
So far, there is no clear evidence that tirzepatide directly harms the kidneys. However, since it is still a new drug, doctors and researchers are watching closely for signs of serious side effects. In clinical trials, some people did report kidney problems, but it is not yet clear whether tirzepatide caused these problems or if they were caused by other health issues. It is also possible that some people were already at risk for kidney trouble before they started the medicine.
Because tirzepatide is still being studied, many people have questions. They want to know how common kidney side effects are, how to spot the signs early, and whether certain people should avoid the medicine. Doctors want to know how to monitor kidney health in people taking tirzepatide and what to do if problems appear.
Understanding the real risk of kidney damage from tirzepatide is important. It helps patients and healthcare providers make better choices. When deciding to use any medicine, it is necessary to weigh the benefits against the risks. Tirzepatide can offer powerful benefits for blood sugar control and weight loss, but it is important to understand its safety profile, especially when it comes to vital organs like the kidneys. This article explores what is currently known about tirzepatide and kidney health. It answers common questions based on clinical trial data, biological mechanisms, and expert recommendations to provide a clear picture of how real the risk of kidney damage might be.
What Is Tirzepatide and How Does It Work?
Tirzepatide is a type of medicine used to treat adults with type 2 diabetes and, more recently, to help with weight loss. It belongs to a group of drugs called incretin mimetics. These drugs copy the actions of certain hormones in the body that help control blood sugar. Tirzepatide is different from many other diabetes medicines because it works on two hormone pathways, not just one.
Tirzepatide activates both the GIP (glucose-dependent insulinotropic polypeptide) and the GLP-1 (glucagon-like peptide-1) receptors. These are two natural hormones found in the gut. When food is eaten, these hormones are released and help regulate blood sugar and appetite.
Most older diabetes medicines that work in this way only target GLP-1. Tirzepatide is the first drug approved that also activates GIP. Because it uses both hormone pathways, tirzepatide may help people lower their blood sugar more effectively and also lose more weight compared to other medicines.
How Tirzepatide Affects Blood Sugar
Tirzepatide helps lower blood sugar in several ways:
- Increases insulin release: After a person eats, blood sugar goes up. Tirzepatide helps the pancreas release more insulin when it’s needed to bring blood sugar down.
- Reduces glucagon: Glucagon is another hormone made by the pancreas. It raises blood sugar when it gets too low. In type 2 diabetes, glucagon levels can be too high. Tirzepatide helps lower glucagon levels after meals.
- Slows down stomach emptying: When the stomach empties more slowly, blood sugar rises more gradually after eating. This helps prevent spikes in blood sugar.
Together, these actions help people with type 2 diabetes keep their blood sugar levels in a healthy range.
How Tirzepatide Affects Weight
Tirzepatide also helps people lose weight. This happens in part because it affects how full and hungry a person feels. After a meal, the GIP and GLP-1 hormones help send signals to the brain that the stomach is full. This reduces appetite and helps people eat less.
People taking tirzepatide often say they feel full sooner and for longer. This can lead to a natural decrease in calorie intake, which can result in weight loss over time.
FDA Approval and Uses
The U.S. Food and Drug Administration (FDA) approved tirzepatide in May 2022 under the brand name Mounjaro. It was first approved to help adults with type 2 diabetes improve their blood sugar levels, along with diet and exercise. In 2023, it was also approved under the brand name Zepbound for weight loss in people with obesity or who are overweight with weight-related health problems.
Tirzepatide is given as a weekly injection under the skin. The dose usually starts low to help the body get used to the medicine. Over time, the dose can be increased based on how well it works and how well it is tolerated.
Tirzepatide has been studied in several large clinical trials. These include the SURPASS studies (for type 2 diabetes) and the SURMOUNT studies (for weight loss). In these trials, people taking tirzepatide showed large improvements in both blood sugar and weight.
For example, in one study, people with type 2 diabetes who took tirzepatide had an average drop in their HbA1c (a measure of long-term blood sugar) of over 2%. This is considered a strong effect. At the same time, many participants also lost 15% or more of their body weight, which is more than what is usually seen with other diabetes or weight-loss drugs.
These benefits make tirzepatide an important new option for people living with type 2 diabetes or obesity. However, as with all medicines, it can have side effects. Some of these side effects may affect the kidneys, which is why kidney safety is being closely studied. Understanding how tirzepatide works helps explain both its benefits and its possible risks.
What Do Clinical Trials Say About Tirzepatide and Kidney Function?
Tirzepatide is a new medication used to treat type 2 diabetes and obesity. It works by activating two types of hormone receptors: GIP and GLP-1. These hormones help lower blood sugar and reduce appetite. While tirzepatide is effective at managing blood sugar and body weight, some people have asked whether it can affect kidney function. To answer this question, it is helpful to look at what clinical trials have found so far.
Clinical Trials Overview
Tirzepatide has been studied in several large clinical trials. These include the SURPASS trials, which tested tirzepatide in people with type 2 diabetes, and the SURMOUNT trials, which focused on weight loss in people with or without diabetes. Thousands of people took part in these studies, and many of them were followed for several months or longer. These trials measured many health outcomes, including blood sugar control, weight loss, heart health, and side effects.
Although kidney function was not the main focus of these trials, researchers still collected some important kidney-related data. For example, they monitored levels of a substance called creatinine in the blood. This helps doctors estimate how well the kidneys are working, a measure called eGFR (estimated glomerular filtration rate). They also looked at urine albumin levels, which can signal early kidney damage.
What the Trials Showed About Kidney Health
In the SURPASS trials, most people who took tirzepatide did not have serious changes in kidney function. The rates of kidney-related side effects were low and were similar to the rates in people taking other diabetes medications, such as insulin or semaglutide. In some patients, researchers even saw slight improvements in kidney markers, especially those who lost a lot of weight or improved their blood sugar control.
In one analysis from SURPASS-4, which included people with higher heart and kidney risk, tirzepatide appeared to slow the decline in eGFR compared to insulin glargine (a long-acting insulin). This suggests that tirzepatide might have a protective effect on the kidneys for some people, although the changes were small. It is important to understand that these findings are early and were not the main goal of the trial. They need to be confirmed in future studies that focus directly on kidney outcomes.
Special Focus on Chronic Kidney Disease (CKD)
Some participants in the SURPASS studies already had chronic kidney disease when they started tirzepatide. These patients were carefully watched for any worsening of kidney function. The results showed that tirzepatide did not cause major kidney problems in these individuals. However, the number of people with advanced kidney disease in these studies was small. That means the results should be interpreted with care.
In people with mild or moderate kidney disease, tirzepatide did not seem to harm kidney function. There was no strong signal of increased risk for acute kidney injury (sudden worsening of kidney function) directly linked to the drug. But some cases of kidney problems did occur, usually in people who also had vomiting, diarrhea, or low blood pressure—conditions that can affect kidney function on their own.
What the Studies Did Not Show
While these trials provided helpful information, they were not designed to answer every question about kidney health. For example, they did not include many people with severe kidney disease or those on dialysis. Also, the trials mostly followed people for a year or less. Kidney damage can take a long time to develop, so longer studies are needed to fully understand the long-term effects.
Most importantly, none of the tirzepatide trials were focused specifically on kidney outcomes. A dedicated kidney outcomes study would be better at showing whether tirzepatide helps or harms the kidneys over time. Some researchers have called for this type of study to be done in the future.
Limitations of the Data
It is important to know the limits of the information available from current clinical trials. First, kidney side effects may be under-reported because they were not the main concern of the studies. Second, the studies did not include all patient types, such as those with severe kidney failure or very low eGFR. Finally, post-marketing data—that is, real-world data from people taking tirzepatide outside of trials—is still being collected.
So far, clinical trials suggest that tirzepatide does not directly harm the kidneys in most people. In some cases, it may even slow kidney decline, especially in people with diabetes who lose weight and improve their blood sugar. However, the data is still early and limited. More research is needed, especially in people with advanced kidney disease and over longer periods of time.

How Common Are Kidney-Related Side Effects with Tirzepatide?
Tirzepatide is a new medication used to treat type 2 diabetes and help with weight loss. Like all medicines, it can cause side effects. Some people have asked if tirzepatide can harm the kidneys. The answer is not simple. Most people taking tirzepatide do not have kidney problems, but a few do. This section explains how often kidney-related side effects happen and what the current studies and reports show.
What Do Clinical Trials Show?
Before tirzepatide was approved, it was tested in large studies called clinical trials. These trials included thousands of people with type 2 diabetes. The most well-known trials were the SURPASS series. In these trials, tirzepatide was compared to other diabetes medicines like insulin or semaglutide. Researchers watched for many side effects, including problems with the kidneys.
In most of these studies, very few people had serious kidney side effects. There were some cases of acute kidney injury (AKI), but they were rare. Acute kidney injury is a sudden loss of kidney function. It can happen for many reasons, including dehydration, infections, or certain medicines. In the SURPASS-4 trial, which included people with higher heart and kidney risk, the number of people who had kidney problems was low and similar to those not taking tirzepatide.
However, most trials were not designed to study kidney safety as a main goal. They were looking at blood sugar control and weight loss. Because of this, kidney side effects may not have been studied closely or followed up for a long time. Also, people with severe kidney disease were not included in most studies. This means there is less information about how tirzepatide affects people with poor kidney function.
What About Real-World Use?
After a medicine is approved, doctors and patients report side effects they see in daily life. These reports are sent to systems like the FDA’s Adverse Event Reporting System (FAERS). These reports can help find side effects that were rare or missed in trials.
In these post-marketing reports, some cases of kidney problems have been seen in people taking tirzepatide. These include reports of AKI and worsening of existing chronic kidney disease (CKD). However, these reports do not always prove that tirzepatide caused the problem. Many people taking tirzepatide already have other health problems like diabetes, high blood pressure, and heart disease — all of which can affect the kidneys.
Also, tirzepatide can cause nausea, vomiting, and diarrhea in some people. These side effects can lead to dehydration. Dehydration lowers the amount of blood flowing to the kidneys and can cause injury, especially in people who already have kidney disease. In some reported cases, people taking tirzepatide became dehydrated and later developed kidney problems. Doctors believe the loss of fluids, not the drug itself, may have been the cause.
How Common Are These Side Effects?
Kidney-related side effects appear to be uncommon. In clinical trials, less than 1% of people had serious kidney problems. Most side effects related to the kidneys were mild or could be reversed. Still, the true number might be slightly higher in real life, where people often have other health conditions and are taking more than one medicine.
The risk seems higher in people who already have kidney disease or who get dehydrated easily. For example, older adults or people taking diuretics (water pills) may be more likely to lose too much fluid. When this happens, their kidneys may not work as well, and problems can occur.
How Does Tirzepatide Compare to Similar Medicines?
Tirzepatide belongs to a group of medicines called GLP-1 receptor agonists. Other drugs in this group include semaglutide and liraglutide. These drugs also have low rates of kidney side effects. Some studies even show they may help protect the kidneys over time. Tirzepatide works a bit differently because it also activates the GIP receptor, but its overall side effect profile is similar.
The good news is that tirzepatide does not seem more dangerous to the kidneys than other similar drugs. In fact, in some studies, it may even slow the worsening of kidney disease in people with diabetes. However, more research is needed to know this for sure.
Kidney-related side effects with tirzepatide are rare but possible. Most often, these problems happen when people lose too much fluid from side effects like vomiting or diarrhea. People with kidney disease, older adults, and those taking other medications should be watched more closely. While the risk is low, doctors should monitor kidney function and watch for warning signs, especially in people with other health issues.
What Are the Biological Mechanisms That Could Link Tirzepatide to Kidney Injury?
Tirzepatide is a medication that helps lower blood sugar and supports weight loss in people with type 2 diabetes or obesity. It works by copying the effects of two natural hormones in the body: GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1). These hormones help control blood sugar, slow down digestion, and reduce appetite. While tirzepatide is helpful for many people, there have been some concerns about how it might affect the kidneys.
Right now, there is no clear proof that tirzepatide directly damages the kidneys. However, some experts think that kidney problems seen in a few people taking tirzepatide may be caused by other effects of the drug. These are called indirect effects. They happen not because the drug is toxic to the kidneys, but because it changes how the body handles fluids, blood pressure, or digestion. Below are the main ways tirzepatide may lead to kidney issues in certain people.
Dehydration from Gastrointestinal Side Effects
One of the most common side effects of tirzepatide is stomach upset. Many people who take the drug report nausea, vomiting, or diarrhea, especially during the first few weeks of treatment. These side effects may lead to dehydration if they continue for too long or are very strong.
The body needs enough fluids to help the kidneys work properly. When someone loses a lot of fluids through vomiting or diarrhea and does not drink enough to replace them, the blood volume can drop. This means there is less blood flowing through the kidneys. The kidneys depend on steady blood flow to filter waste and make urine. If blood flow drops too much, the kidneys may start to work poorly. In serious cases, this can lead to acute kidney injury (AKI), a condition where the kidneys suddenly stop working well.
People with pre-existing kidney problems, older adults, and those taking other medications that affect fluid balance may be more sensitive to dehydration. For these groups, even mild stomach problems could cause a drop in kidney function.
Lower Blood Pressure and Reduced Kidney Perfusion
Tirzepatide can cause weight loss and lower blood pressure, which are usually good things for people with type 2 diabetes. However, if blood pressure drops too low or too quickly, it may reduce blood flow to the kidneys. This is especially a concern in people who already have low blood pressure, heart problems, or poor circulation.
The kidneys rely on healthy blood pressure to get the oxygen and nutrients they need. If blood pressure drops too much, the kidneys may not get enough blood. This condition, called hypoperfusion, can stress the kidneys and sometimes lead to damage over time. In most people, this does not happen, but in those who are already at risk, it is something to watch closely.
Volume Depletion and Electrolyte Imbalance
In addition to dehydration and low blood pressure, volume depletion can occur. This means that the total amount of fluid in the body drops too low. When fluid levels are low, the body tries to hold onto water and salt, which can affect how the kidneys function. The kidneys may reduce how much urine they make, and waste products may build up in the blood.
Also, with vomiting or diarrhea, the body can lose important salts such as potassium and sodium. These salts, called electrolytes, help control how muscles and nerves work and keep the kidneys working properly. If electrolytes become imbalanced, the kidneys may struggle even more.
No Evidence of Direct Kidney Toxicity
Unlike some medications that are known to be toxic to the kidneys, there is currently no strong evidence that tirzepatide itself harms kidney cells directly. Lab studies and clinical trials have not shown that tirzepatide causes structural damage to the kidneys. Instead, most kidney problems reported so far seem to be related to the indirect effects listed above—such as dehydration and low blood pressure—rather than direct injury to the kidney tissue.
This is important because it means that in most people, tirzepatide does not seem to pose a high risk to the kidneys on its own. But if someone taking tirzepatide becomes very dehydrated, sick, or already has poor kidney function, the chances of kidney-related problems may go up.
Interaction with Other Medications
Many people who take tirzepatide are also taking other medications, including drugs for blood pressure, diabetes, or pain. Some of these medications can also affect kidney function. For example, non-steroidal anti-inflammatory drugs (NSAIDs), certain blood pressure medicines (like ACE inhibitors or diuretics), and contrast dyes used in imaging tests can increase the risk of kidney injury.
When tirzepatide is added to these drugs, especially in someone with poor fluid intake or vomiting, the risk of kidney injury may increase further. Doctors need to look at the full list of a person’s medications to make sure they are not adding to the risk.
Tirzepatide does not appear to damage the kidneys directly. However, it can lead to indirect problems that may affect kidney health, especially in vulnerable people. These include dehydration from vomiting or diarrhea, low blood pressure, reduced blood flow to the kidneys, and loss of important fluids and electrolytes. When used carefully and with proper monitoring, tirzepatide is generally safe for the kidneys. But people with existing kidney problems or other risk factors should be watched more closely during treatment.
Who May Be at Greater Risk of Kidney Problems While Taking Tirzepatide?
Tirzepatide is a medicine that helps lower blood sugar and support weight loss. While it is helpful for many people, some may be at higher risk of kidney problems when using it. Understanding who may be more vulnerable can help doctors and patients take extra care. Several factors can raise the chance of kidney issues, especially in people who already have other health problems.
People With Pre-Existing Kidney Disease
Individuals who already have chronic kidney disease (CKD) are more likely to experience further kidney problems with any new medication. CKD means that the kidneys are not working as well as they should. Tirzepatide has not been shown to directly harm the kidneys, but people with CKD already have reduced kidney function. Even a small change in hydration, blood pressure, or other body systems can cause their kidney health to get worse.
For these individuals, small changes such as dehydration from vomiting or diarrhea (common side effects of tirzepatide) can cause a sudden drop in kidney function. This is known as acute kidney injury (AKI). People with stage 3 or higher CKD must be watched more closely if they start tirzepatide.
Older Adults
Older adults are at greater risk because aging naturally lowers kidney function. Many people over age 65 already have some level of kidney decline, even if they do not feel sick. Aging also makes it harder for the body to handle fluid changes and stress. When older adults take tirzepatide, they may not drink enough water if they feel nauseated or full, which can lead to dehydration. Their kidneys may struggle to keep up, especially if other health problems are present.
Older people are also more likely to take several medications at once. Some of these medicines can also affect the kidneys or body fluids, which increases the risk of a problem when tirzepatide is added.
People Taking Other Medications That Affect the Kidneys
Certain medicines can stress the kidneys. Examples include diuretics (water pills), non-steroidal anti-inflammatory drugs (NSAIDs) like ibuprofen, and some blood pressure drugs like ACE inhibitors or ARBs. These medications, when taken along with tirzepatide, may increase the chances of kidney injury.
For example, diuretics make the body lose water through urine. If tirzepatide also causes vomiting or diarrhea, the body may lose too much water. This can lower blood pressure and reduce the amount of blood reaching the kidneys. When that happens, kidney function may suddenly decline.
Doctors need to review all the medications a person is taking before starting tirzepatide, especially in people with other risk factors. Adjustments may be needed to lower the risk.
People With Heart Failure
Heart failure can reduce the flow of blood to the kidneys. In these cases, the kidneys may already be under stress. People with heart failure often take diuretics or other medications that change body fluids or blood pressure. Tirzepatide may add to this effect. If too much fluid is lost, kidney function may worsen. These individuals need careful monitoring.
People With Low Fluid Intake or Dehydration
Tirzepatide may cause side effects like nausea, vomiting, or diarrhea, especially when treatment begins. These effects can make people eat and drink less. If they do not take in enough fluids, they may become dehydrated. Dehydration reduces the amount of blood that flows through the kidneys. Without enough blood flow, the kidneys cannot filter waste properly, and this can lead to AKI.
People who are already not drinking enough water—either due to illness, heat, or diet—are more likely to have problems when taking tirzepatide. Encouraging fluid intake is an important step in prevention.
People With a History of Kidney Injury
Individuals who have had kidney injury in the past may be more likely to have it again. The kidneys can become more sensitive after a previous injury. Even if kidney function returned to normal, these people should be monitored closely when starting tirzepatide.
People With Low Blood Pressure
Tirzepatide may lower blood pressure. For some people, this can be a good thing. But for others, especially those with already low blood pressure or who take medicine that lowers it, this can reduce the blood supply to the kidneys. Low blood pressure can lead to poor kidney perfusion, which may cause injury over time. People who often feel dizzy or lightheaded, especially when standing up, may be at higher risk.
People with kidney disease, older adults, those taking certain medications, and individuals with conditions like heart failure are more likely to face kidney problems with tirzepatide. Others who do not drink enough fluids, who have had kidney injury before, or who have low blood pressure may also be at risk. Doctors usually check kidney function before starting tirzepatide and monitor it regularly, especially in those who have any of these risk factors.
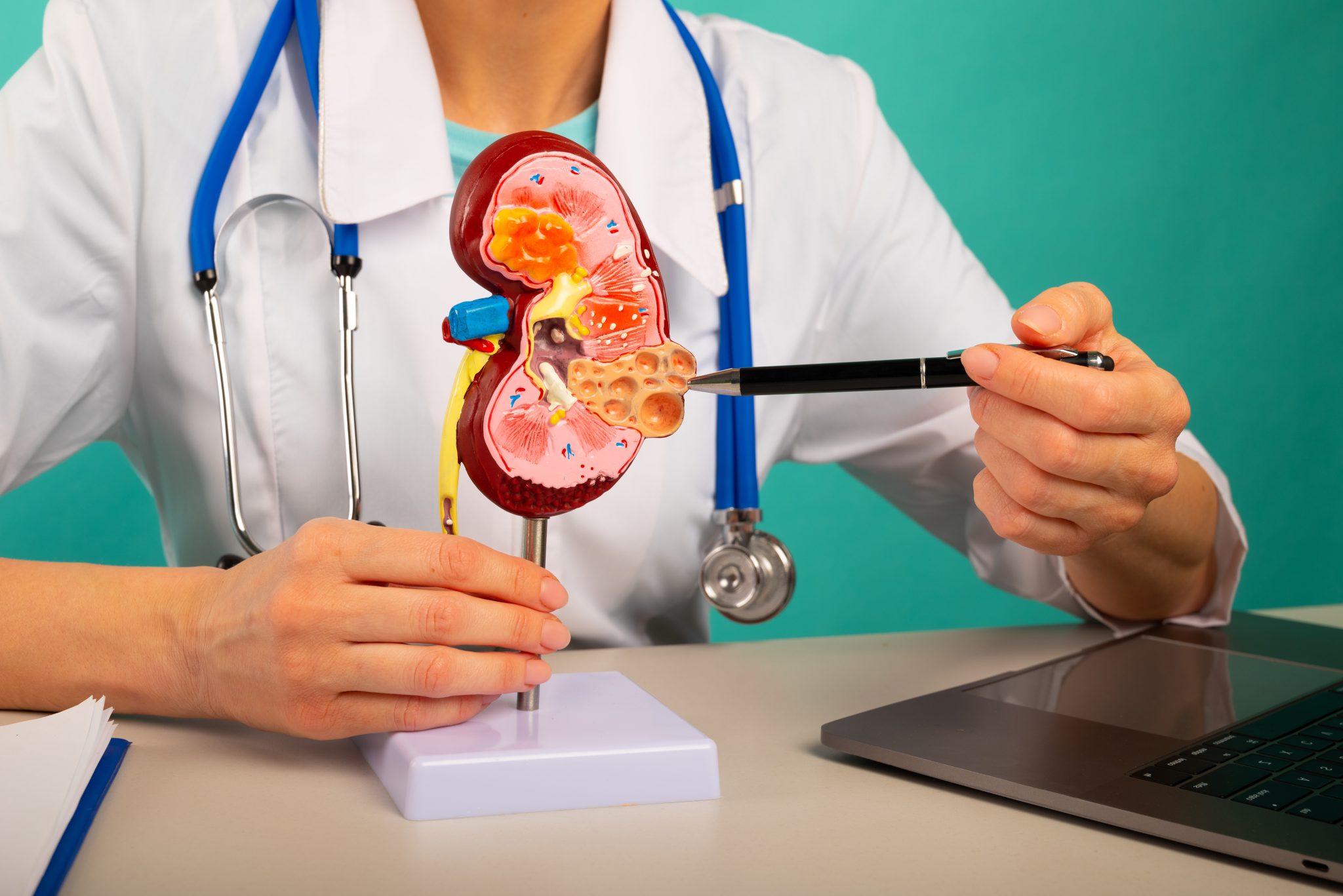
Can Tirzepatide Be Safely Used in People with Chronic Kidney Disease?
Tirzepatide is a newer medication used to manage type 2 diabetes and obesity. Many people with type 2 diabetes also have chronic kidney disease (CKD), which makes it important to understand whether tirzepatide is safe for them. CKD happens when the kidneys slowly lose their ability to filter waste and fluids from the blood. This can lead to serious health problems if not managed properly.
What the FDA Label Says
The U.S. Food and Drug Administration (FDA) has approved tirzepatide for adults with type 2 diabetes and for weight loss in those who are overweight or obese with at least one weight-related condition. The current prescribing information does not list any specific dose changes based on kidney function. This means tirzepatide can be used without adjusting the dose in people with mild, moderate, or even severe kidney disease. However, it has not been tested enough in people with end-stage kidney disease (ESKD) or those on dialysis, so its safety in those groups is not known.
The FDA labeling states that no dose adjustment is needed for people with an estimated glomerular filtration rate (eGFR) as low as 15 mL/min/1.73 m², which is considered severe kidney disease. Even so, doctors are told to use caution in patients with poor kidney function, especially when there is a risk of dehydration.
What Doctors Recommend
Endocrinologists and nephrologists—doctors who specialize in hormones and kidney disease—often recommend being extra careful when prescribing tirzepatide to people with CKD. Although there is no evidence that tirzepatide directly harms the kidneys, the drug can cause nausea, vomiting, and diarrhea. These side effects may lead to dehydration, which can be dangerous in people with kidney problems. When the body loses too much fluid, the kidneys do not get enough blood flow, which can cause further damage or even acute kidney injury (AKI).
Doctors may start with the lowest possible dose and slowly increase it if the patient handles it well. They also monitor kidney function regularly, especially during the first few weeks of treatment or when side effects occur. Patients who are at high risk for dehydration may be given advice about how to stay hydrated and when to call a doctor.
Dosing and Kidney Function
Tirzepatide is given by injection once a week. It comes in several dose strengths, starting at 2.5 mg and going up to 15 mg. The dose is usually increased over time to help reduce side effects. People with CKD do not need a different starting dose, but doctors often choose to be more careful when adjusting the dose.
There are no specific instructions for dose changes based on eGFR levels. Still, if a patient has worsening kidney function or shows signs of dehydration, the doctor may pause the medication or lower the dose until things improve.
What the Clinical Trials Show
In the large clinical studies that led to tirzepatide’s approval, some patients with kidney disease were included. The studies did not show any major increase in kidney problems compared to other diabetes medicines. In fact, some early data suggest that tirzepatide may even help slow down kidney damage in people with diabetes, similar to what has been seen with GLP-1 receptor agonists. However, more research is needed to know this for sure.
These early findings are promising, but it’s important to remember that people with the most severe kidney disease were not well represented in the trials. This makes it harder to say how safe tirzepatide really is for people on dialysis or those with advanced kidney failure.
Tirzepatide can be used in people with chronic kidney disease without changing the dose, as long as they are not on dialysis. Doctors should be careful in patients with poor kidney function, especially if they have other risks like heart failure or are taking water pills (diuretics). The main concern is dehydration from side effects, not direct harm to the kidneys. Regular monitoring and patient education are key to safe use in this group.
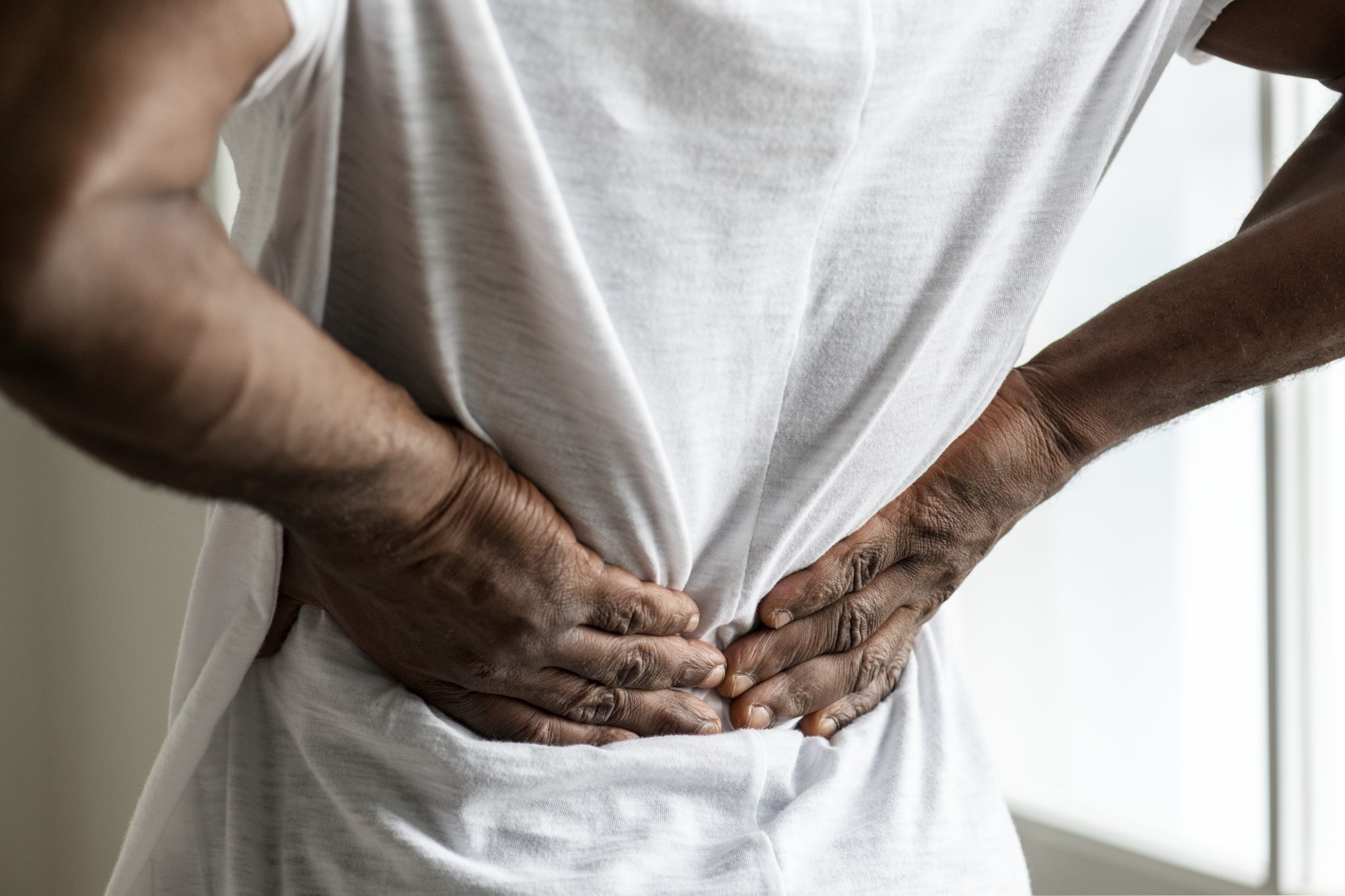
What Are the Signs and Symptoms of Kidney Problems Patients Should Watch For?
Kidney problems can sometimes be hard to notice at first. Many people with kidney issues do not feel sick until the condition becomes more serious. That is why it is important to know the early signs and symptoms that may point to a kidney problem, especially for those taking medications like tirzepatide. Even though most people taking tirzepatide do not have kidney side effects, a small number may develop issues such as dehydration or, in rare cases, acute kidney injury (AKI).
Tirzepatide may affect the kidneys indirectly. This can happen if the medicine causes vomiting, diarrhea, or poor appetite. These side effects may lead to dehydration. Dehydration lowers the amount of fluid in the body. When the body loses too much fluid, the kidneys may not get enough blood flow. This can put stress on the kidneys and reduce their ability to filter waste.
Early Signs of Kidney Problems
Some early warning signs that the kidneys may not be working well include:
- Fatigue or feeling very tired: When the kidneys are not working properly, waste builds up in the blood. This can make the body feel weak, tired, or have trouble concentrating.
- Swelling in the legs, ankles, or feet: When the kidneys do not remove extra fluid from the body, it can build up. Swelling, also called edema, is often one of the first physical signs of a kidney issue.
- Changes in urination: The kidneys control how much urine the body makes. A person may notice they are urinating more often, especially at night, or that they are making less urine than usual. The color of urine may also change—becoming darker, foamy, or bloody.
- Shortness of breath: Extra fluid in the body can build up in the lungs. This can make breathing harder, especially during physical activity or when lying down.
- Loss of appetite, nausea, or metallic taste in the mouth: As waste builds up in the blood, it can affect the digestive system and sense of taste. These symptoms can also be related to side effects of tirzepatide but should not be ignored if they continue or get worse.
Warning Signs of Acute Kidney Injury (AKI)
Acute kidney injury means the kidneys stop working suddenly, usually over a few hours or days. This condition can become serious quickly and needs medical attention. The signs of AKI may include:
- Very little or no urine output
- Rapid swelling in the face, hands, or legs
- Sudden confusion or drowsiness
- Severe nausea and vomiting that does not improve
- Pain or pressure in the lower back (where the kidneys are located)
- High blood pressure that suddenly worsens
In many cases, AKI happens because of dehydration, low blood pressure, or use of other medicines that affect the kidneys. People taking tirzepatide who also take water pills (diuretics), blood pressure medicines like ACE inhibitors, or pain relievers like NSAIDs may be at higher risk.
When to Seek Medical Attention
It is important to speak to a healthcare provider if any of the signs listed above appear, especially if they last more than a day or two. A doctor may order blood or urine tests to check kidney function. These tests can find problems early, even before symptoms become obvious.
Getting help early can prevent a minor issue from becoming a more serious problem. In some cases, stopping or adjusting the medicine may be enough to protect the kidneys. A healthcare provider will decide if changes in treatment are needed based on the person’s overall health, kidney function, and symptoms.
Being aware of how the body feels and noticing any new or unusual symptoms can help catch kidney problems early. This is especially important for people who already have risk factors for kidney disease, such as high blood pressure, diabetes, or a history of kidney issues. Regular follow-up and lab checks during treatment with tirzepatide are an important part of staying safe.
How Should Kidney Function Be Monitored During Tirzepatide Therapy?
Monitoring kidney function is important for anyone taking tirzepatide, especially those with risk factors for kidney problems. Although tirzepatide is not directly harmful to the kidneys in most cases, it can affect the body in ways that may lead to kidney stress or damage. Careful and regular testing helps detect changes early and prevents more serious complications.
Tests to Check Kidney Function
The main tests used to check kidney health are simple blood and urine tests. These include:
- Serum Creatinine: This is a blood test that measures the level of creatinine, a waste product from muscles. Healthy kidneys remove creatinine from the blood. If levels are high, it may mean the kidneys are not working well.
- Estimated Glomerular Filtration Rate (eGFR): This number is calculated based on the creatinine level, age, sex, and other factors. It shows how well the kidneys are filtering the blood. An eGFR below 60 may suggest chronic kidney disease.
- Blood Urea Nitrogen (BUN): This test measures another waste product in the blood. High BUN levels can also be a sign of poor kidney function, especially when combined with high creatinine.
- Urine Albumin-to-Creatinine Ratio (UACR): This urine test checks for protein (albumin) in the urine. A small amount of protein may be normal, but high levels can signal kidney damage.
When to Check Kidney Function
Before starting tirzepatide, it is recommended to check kidney function to make sure it is safe to begin treatment. This includes doing a baseline test for creatinine, eGFR, and urine albumin.
After treatment begins, how often kidney function is tested depends on the person’s overall health and risk level.
For People with Normal Kidney Function:
- Kidney function should be checked at least once a year.
- If there are no symptoms or health problems, yearly monitoring is usually enough.
For People with Higher Risk of Kidney Problems:
- Testing may need to be done more often, every 3 to 6 months.
- People with diabetes, high blood pressure, or mild kidney disease should be watched more closely.
- Anyone taking other medications that affect the kidneys, such as NSAIDs or diuretics, may also need more frequent testing.
What Triggers More Frequent Monitoring?
Certain situations may increase the need to check kidney function more often:
- Dehydration: Tirzepatide can cause nausea, vomiting, or diarrhea in some people, leading to fluid loss. Dehydration can reduce kidney blood flow and raise the risk of acute kidney injury (AKI).
- Poor Oral Intake: If someone is not eating or drinking well while on tirzepatide, this may affect kidney function.
- Sudden Changes in Health: New symptoms like fatigue, swelling, reduced urine output, or confusion could point to kidney stress.
- Recent Illness or Infection: Fever, vomiting, or other infections can stress the kidneys, especially if combined with tirzepatide.
- Changes in Other Medications: Adding or increasing doses of medicines like blood pressure drugs, diuretics, or pain relievers may impact the kidneys.
When any of these issues come up, doctors may repeat kidney function tests sooner than planned.
How to Prepare for Tests
Most blood tests for kidney function do not require fasting. Drinking water before a blood draw is helpful, especially if dehydration is a concern. Urine tests may involve collecting a sample at a clinic or doing a home collection.
Why Monitoring Matters
Keeping track of kidney health helps doctors adjust treatment early if needed. If test results show worsening kidney function, tirzepatide may be paused or stopped. In some cases, doctors may reduce the dose or make changes to other medications to protect the kidneys.
Monitoring also gives a clearer picture of how tirzepatide is affecting the body over time. Even if no problems are seen at first, regular testing can catch small changes before they become serious.
Early detection and action make a big difference in preventing long-term kidney damage. By following a regular testing schedule, both patients and healthcare providers can keep treatment safe and effective.
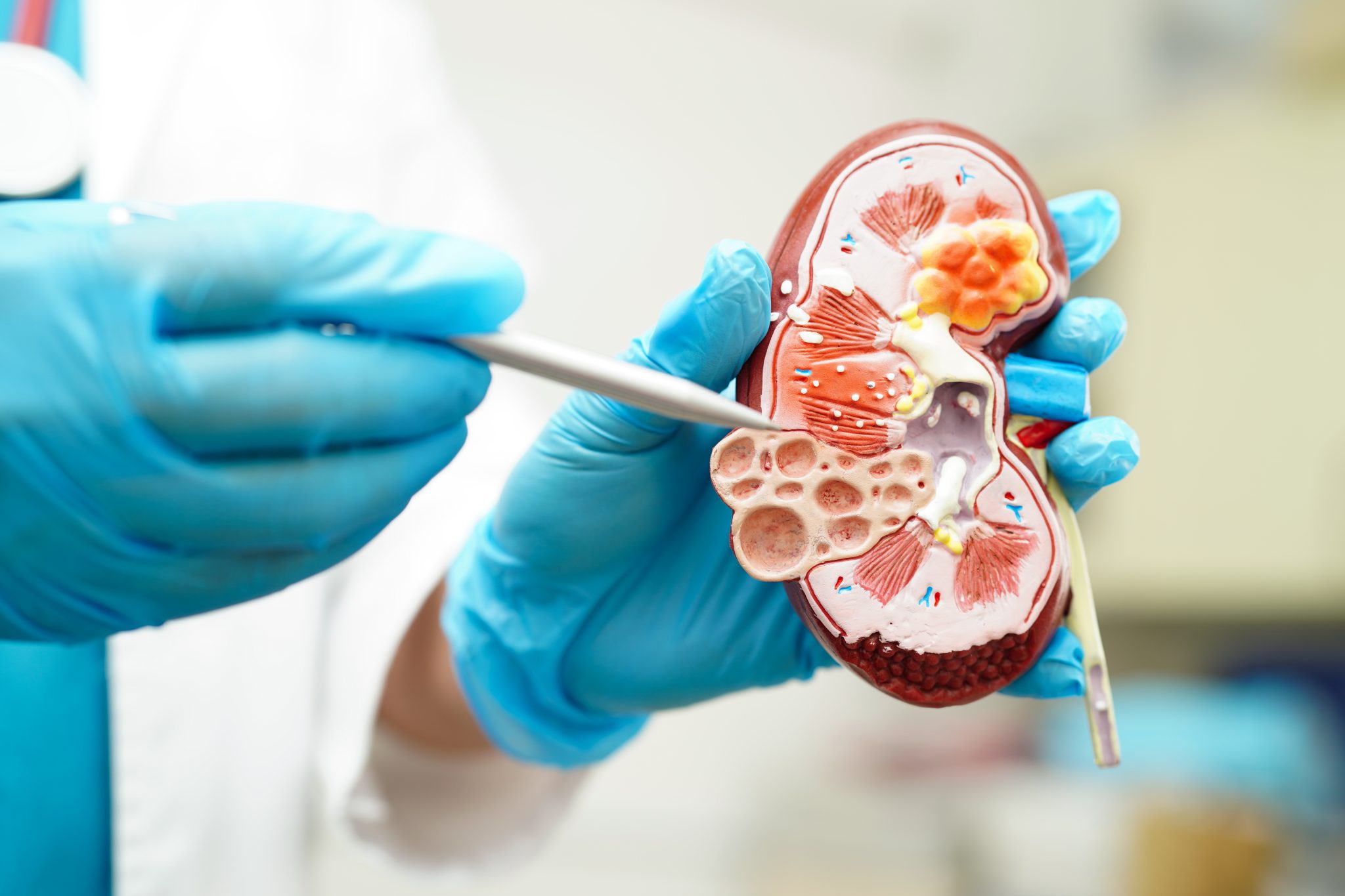
What Should Clinicians Do if Kidney Injury Is Suspected During Tirzepatide Use?
When a person taking tirzepatide shows signs of possible kidney problems, doctors need to act quickly and carefully. Kidney injury can sometimes develop slowly, but it can also come on suddenly. Taking early steps can help prevent serious complications and protect kidney function.
Stop the Medication Right Away
If there are any signs of kidney injury, stopping tirzepatide is usually the first step. Tirzepatide is not known to directly harm the kidneys. However, it can cause side effects like nausea, vomiting, or diarrhea. These can lead to dehydration, which may reduce blood flow to the kidneys and cause damage. When this happens, the kidneys may not filter blood properly. Taking the medicine off temporarily gives the body a chance to recover.
The doctor will also ask about how much fluid the person has been drinking, if they have had vomiting or diarrhea, and if they have felt weak or dizzy. These symptoms often suggest that the person may be dehydrated. Dehydration can lower blood pressure and reduce blood flow to the kidneys.
Check Volume Status and Blood Pressure
One of the next steps is to check the person’s fluid balance. This means looking for signs that the body may not have enough fluids. Dry mouth, low blood pressure, fast heart rate, and little urine output are common signs of dehydration. Sometimes, people also lose their normal thirst response, especially if they are older or have other medical problems.
Blood pressure should be measured carefully. Low blood pressure may make kidney injury worse. In some cases, doctors may give fluids through a vein (IV) to help improve blood flow to the kidneys and prevent further injury.
Review Other Medicines
Tirzepatide is often used with other drugs for diabetes or high blood pressure. Some of these medications may also affect kidney function. For example:
- ACE inhibitors or ARBs (used to treat high blood pressure) can lower kidney blood flow.
- NSAIDs like ibuprofen or naproxen can also reduce kidney function if used too often.
- Diuretics (water pills) can lead to fluid loss, especially when combined with vomiting or diarrhea.
If someone has kidney problems while on tirzepatide, the doctor will review all the medications being taken. The goal is to stop or adjust drugs that may make the problem worse. This can give the kidneys a better chance to heal.
Order Kidney Function Tests
To confirm a kidney injury, blood and urine tests are needed. Blood tests may include:
- Serum creatinine – a waste product that rises when kidney function drops.
- Blood urea nitrogen (BUN) – another waste product that can go up when the kidneys aren’t working well.
- Estimated glomerular filtration rate (eGFR) – a number that shows how well the kidneys are filtering blood.
Urine tests may also be ordered to look for protein, blood, or other signs of damage. Doctors may also check urine output over time to see if the kidneys are making enough urine.
Rule Out Other Causes
Not all kidney injuries are caused by tirzepatide. Doctors must check for other possible causes such as:
- Infections
- Blockages in the urinary tract
- Severe high blood pressure
- Heart problems that affect kidney function
A full check-up is important so that the correct treatment is given. In some cases, imaging tests like a kidney ultrasound may be needed to look for any physical problems.
Decide on Restarting Tirzepatide
Once the kidney problem is under control, the doctor may decide whether or not to restart tirzepatide. This decision depends on what caused the kidney injury and how well the kidneys recovered. If dehydration or other reversible causes were the reason, it may be safe to restart the medicine at a lower dose. However, careful monitoring will be needed.
Some people may need to stop the medicine completely, especially if their kidneys do not fully recover or if the risk of another injury is high.
Refer to a Kidney Specialist When Needed
If kidney function does not improve after stopping tirzepatide and giving supportive care, referral to a nephrologist (kidney doctor) may be necessary. A specialist can do more testing to find out why the kidneys are not working properly and recommend long-term treatment options.
People with existing chronic kidney disease (CKD) who develop sudden kidney problems should also be seen by a nephrologist. Early referral can help slow down further damage and improve long-term outcomes.
By acting quickly when kidney problems are suspected, healthcare providers can reduce harm and improve recovery. Knowing when to stop the medication, check fluids and other medicines, and monitor kidney function plays a key role in protecting patients from more serious kidney issues.
Are There Any Regulatory Warnings or Safety Alerts About Tirzepatide and Kidneys?
Tirzepatide is a newer medicine approved by the U.S. Food and Drug Administration (FDA) for type 2 diabetes. It is also being studied and used for weight loss. Like any medicine, it goes through a careful approval process where safety is reviewed. This includes looking for possible risks to the kidneys. So far, no formal warnings or safety alerts have been issued by major health agencies like the FDA or the European Medicines Agency (EMA) about tirzepatide causing direct kidney damage. However, monitoring continues as more people use the drug in real-world settings.
FDA Review and Safety Information
The FDA approved tirzepatide in 2022 under the brand name Mounjaro for adults with type 2 diabetes. During the approval process, the FDA reviewed data from large clinical trials. These trials included patients with and without kidney problems. Some patients did have mild changes in kidney function, but most changes were linked to fluid loss from vomiting or diarrhea. These side effects are common with GLP-1 receptor agonists, a group of drugs that includes tirzepatide.
The FDA label for tirzepatide includes a section on kidney function. It states that there have been reports of acute kidney injury (AKI) in patients using similar drugs, especially in those who experienced severe nausea, vomiting, or diarrhea. The label explains that these side effects can lead to dehydration. Dehydration may reduce blood flow to the kidneys, which could cause kidney injury in some people. The warning encourages doctors to monitor patients who are at higher risk of kidney problems. It also advises that kidney function be watched more closely if the patient has a history of kidney disease or is taking medications that affect the kidneys.
Even though the FDA has not issued a black box warning for tirzepatide related to kidney injury, the product label makes it clear that caution is needed in certain cases.
European Medicines Agency (EMA) Position
The European Medicines Agency, which regulates drugs in Europe, approved tirzepatide in 2022 as well. The EMA has not published any specific safety alerts related to kidney problems from tirzepatide. Like the FDA, the EMA includes information about possible kidney-related side effects in the official prescribing documents. These documents advise healthcare professionals to monitor kidney function in people who may be more likely to have kidney issues. The guidance is especially important for older adults and those taking diuretics or blood pressure medications.
So far, the EMA has not raised concerns beyond those already stated during the approval process. However, the agency collects reports of side effects through its EudraVigilance system, which keeps track of new problems that may arise once the drug is used widely.
Post-Marketing Surveillance and Real-World Use
Post-marketing surveillance is an important part of drug safety. It helps find rare or delayed side effects that may not show up in clinical trials. Both the FDA and EMA rely on doctors, pharmacists, and patients to report any serious problems through programs like the FDA’s MedWatch or the EMA’s EudraVigilance.
Since tirzepatide is still a relatively new medication, post-marketing data is still being collected. As of now, there have been no widespread reports of kidney damage directly linked to the drug. However, some individual cases of acute kidney injury have been reported. In most of these reports, the patients also had vomiting, diarrhea, or low fluid intake, which can all lower kidney function. This suggests the kidney problems may not be caused by the drug itself, but by dehydration related to side effects.
Ongoing Research and Monitoring
Regulatory agencies continue to monitor the safety of tirzepatide through ongoing studies. Long-term data is still being collected to understand how the drug affects the kidneys over time. Studies such as SURPASS-CVOT, which examines heart and kidney outcomes, will provide more detailed information in the future.
The manufacturers of tirzepatide are also required to report safety updates to health agencies regularly. These updates include summaries of new side effects reported by healthcare providers or seen in further studies.
There are currently no formal warnings or safety alerts from the FDA or EMA that say tirzepatide directly causes kidney damage. However, both agencies recognize that the drug may cause problems in people who become dehydrated from nausea, vomiting, or diarrhea. As a result, healthcare providers are advised to monitor kidney function, especially in people who are at higher risk. Ongoing research and post-marketing data will help determine if more warnings are needed in the future.
Conclusion
Tirzepatide is a medication used to help people with type 2 diabetes and obesity. It works by copying two hormones in the body: GIP and GLP-1. These hormones help control blood sugar and make people feel full. Tirzepatide has helped many people lower their blood sugar and lose weight. Because of this, it has become more common in medical treatment. But as more people use tirzepatide, some questions have come up about how safe it is, especially for the kidneys.
So far, the risk of kidney damage from tirzepatide appears to be low. There is no strong proof that tirzepatide directly harms the kidneys. In clinical trials, most people taking the drug did not have serious kidney problems. Still, some cases of kidney-related side effects have been reported. These include dehydration, changes in kidney function, and, in rare cases, acute kidney injury. These problems may not be caused by the drug itself. Instead, they may be linked to other things that happen while taking the drug, like vomiting or diarrhea, which can lead to a loss of fluids.
The way tirzepatide might affect the kidneys seems to be mostly indirect. This means the drug does not damage the kidneys itself. But it can cause side effects, such as nausea, vomiting, or diarrhea. These side effects can make it hard for a person to keep enough fluid in the body. When the body is low on fluids, the kidneys have to work harder. If this continues, the kidneys may become injured. People who are already sick, older, or taking other drugs that stress the kidneys may have a higher risk.
Certain groups of people may need to be more careful. Those with long-term kidney disease, heart failure, or who take water pills or other strong medications should be watched closely. These people may have a harder time handling the loss of fluids or changes in blood pressure. Doctors may need to check their kidney function more often or adjust how the medicine is used. There are no current rules that say the dose of tirzepatide must be changed based on kidney health, but doctors still need to be cautious.
People taking tirzepatide should also know the warning signs of kidney trouble. Feeling very tired, having swelling in the legs, or peeing less than usual may be early signs that the kidneys are not working well. If these signs show up, the doctor may need to stop the drug or do more tests. Blood tests can check how well the kidneys are working. These tests may include checking creatinine, eGFR, and looking for protein in the urine. Keeping an eye on these numbers can help doctors find problems early.
So far, there have not been major warnings from drug safety agencies like the FDA about tirzepatide and kidney failure. Still, reports of kidney side effects after the drug was approved are being studied. As more people use tirzepatide, doctors and researchers will learn more about how it affects kidney health over time. This is why ongoing research is so important. Right now, the studies show that serious kidney problems are rare and usually happen when other risk factors are present.
In summary, tirzepatide does not seem to cause kidney damage directly. Most people can use it safely, especially if they are in good health and drink enough fluids. The risk of kidney injury may rise in people who are older, have kidney disease already, or get very sick from vomiting or diarrhea. For these people, it is important to monitor kidney function and stay well hydrated. Doctors should make decisions based on each person’s full health picture. The balance between the drug’s benefits and its risks must be weighed carefully. More long-term studies are still needed to fully understand how tirzepatide affects kidney function over many years.
Research Citations
Heerspink, H. J. L., Sattar, N., Pavo, I., Haupt, A., Duffin, K. L., Yang, Z., Wiese, R. J., Tuttle, K. R., & Cherney, D. Z. I. (2022). Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: Post-hoc analysis of an open-label, randomised, phase 3 trial. The Lancet Diabetes & Endocrinology, 10(11), 774–785. doi:10.1016/S2213-8587(22)00243-1
Bosch, C., Carriazo, S., Soler, M. J., Ortiz, A., & Fernandez-Fernandez, B. (2022). Tirzepatide and prevention of chronic kidney disease. Clinical Kidney Journal, 16(5), 797–808. doi:10.1093/ckj/sfac274
Caruso, I., & Giorgino, F. (2024). Renal effects of GLP-1 receptor agonists and tirzepatide in individuals with type 2 diabetes: Seeds of a promising future. Endocrine, 84, 822–835. doi:10.1007/s12020-024-03757-9
Kamrul-Hasan, A., Patra, S., Dutta, D., Nagendra, L., Muntahi-Reza, A., Borozan, S., & Pappachan, J. M. (2025). Renal effects and safety of tirzepatide in subjects with and without diabetes: A systematic review and meta-analysis. World Journal of Diabetes, 16(2), 101282. doi:10.4239/wjd.v16.i2.101282
Heerspink, H. J. L., Sattar, N., Pavo, I., Haupt, A., Duffin, K. L., Yang, Z., Wiese, R. J., Wilson, J. M., Hemingway, A., Cherney, D. Z. I., & Tuttle, K. R. (2023). Effects of tirzepatide versus insulin glargine on cystatin C–based kidney function: A SURPASS-4 post hoc analysis. Diabetes Care, 46(8), 1501–1506. doi:10.2337/dc23-0261
Kosaraju, S. A., & Zhang, R. M. (2024). Tirzepatide prescribing practices and efficacy in patients with diabetes and chronic kidney disease at a large tertiary care center in the United States. Diabetes, Metabolic Syndrome and Obesity, 17, 3621–3628. doi:10.2147/DMSO.S473319
Chuang, M.-H., Chen, J.-Y., Wang, H.-Y., Jiang, Z.-H., & Wu, V.-C. (2024). Clinical outcomes of tirzepatide or GLP-1 receptor agonists in individuals with type 2 diabetes. JAMA Network Open, 7(8), e2427258. doi:10.1001/jamanetworkopen.2024.27258
Bradley, C. L., McMillin, S. M., Hwang, A. Y., & Sherrill, C. H. (2023). Tirzepatide, the newest medication for type 2 diabetes: A review of the literature and implications for clinical practice. The Annals of Pharmacotherapy, 57(7), 822–836. doi:10.1177/10600280221134127
Dahl, D., Onishi, Y., Norwood, P., Odell, M., Cao, D., & Frias, J. P. (2022). Effect of subcutaneous tirzepatide versus placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: The SURPASS-5 randomized clinical trial. JAMA, 327(6), 534–545. doi:10.1001/jama.2022.0143
Frias, J. P., Nauck, M. A., Van J., Kutney, G. M., To, W., Lindberg, S., Milicevic, Z., & Del Parigi, A. (2021). LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Molecular Metabolism, 43, 101103. doi:10.1016/j.molmet.2020.101103
Questions and Answers: Tirzepatide Kidney Damage
Tirzepatide is a medication used to treat type 2 diabetes and obesity. It acts as a dual GLP-1 and GIP receptor agonist to help control blood sugar and support weight loss.
Tirzepatide is not known to directly cause kidney damage. However, there have been rare reports of acute kidney injury (AKI), often in the context of dehydration or preexisting kidney conditions.
Tirzepatide may indirectly impact kidney function by causing side effects like nausea, vomiting, or diarrhea, which can lead to dehydration. Dehydration can, in turn, contribute to reduced kidney perfusion and possible acute kidney injury.
Patients with chronic kidney disease (CKD) may be more susceptible to adverse effects like dehydration, but tirzepatide is generally considered safe for use in mild to moderate CKD with proper monitoring.
Yes, clinical trials of tirzepatide have included patients with varying degrees of kidney function. Results suggest that it may slow progression of kidney disease, particularly by improving glycemic control and reducing albuminuria.
Signs include decreased urine output, swelling in the legs or ankles, fatigue, confusion, and shortness of breath. These may indicate fluid retention or worsening kidney function.
Yes, it’s recommended to monitor kidney function, especially in patients with preexisting kidney issues or those experiencing significant gastrointestinal side effects.
Emerging data suggest tirzepatide may have renal benefits similar to other GLP-1 receptor agonists, such as reducing albuminuria and improving blood sugar and blood pressure, which are risk factors for kidney disease progression.
They should stay well-hydrated and contact their healthcare provider. Persistent gastrointestinal symptoms can lead to dehydration and negatively affect kidney function.
Tirzepatide is not currently approved for use in patients with end-stage renal disease (ESRD) or those on dialysis, due to limited safety data in this population.