Table of Contents
Introduction
Tirzepatide is a medication that has changed how doctors treat type 2 diabetes and obesity. Sold under brand names such as Mounjaro and Zepbound, this medicine helps lower blood sugar levels and supports significant weight loss. For many people, tirzepatide has brought new hope, especially for those who struggled with managing diabetes or reducing weight through other treatments. However, as more patients have begun using it, pharmacies and clinics across the United States and other countries have started facing a major problem — a shortage of tirzepatide.
In recent months, many patients have gone to refill their prescriptions only to find that their pharmacy shelves are empty. Some pharmacies have placed patients on long waiting lists, while others have received only small shipments that sell out in hours. Healthcare providers are getting daily calls from worried patients asking what they should do. This growing shortage has led to widespread concern, not just among patients, but also among doctors, pharmacists, and public health officials.
To understand this shortage, it is important to know why tirzepatide became so popular so quickly. Tirzepatide works by acting on two natural hormones in the body called GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotropic polypeptide). These hormones help control blood sugar and appetite. By mimicking their effects, tirzepatide helps the body use insulin more effectively and reduces hunger, which can lead to both better blood sugar control and weight loss. The combination of these effects made tirzepatide unique compared to older diabetes medicines.
After its approval by the U.S. Food and Drug Administration (FDA) for type 2 diabetes in 2022, demand for tirzepatide grew quickly. When clinical trials later showed its strong effect on weight loss, interest exploded even more — not just among people with diabetes, but also those seeking treatment for obesity. In late 2023, the FDA approved tirzepatide under the brand name Zepbound for chronic weight management. By that point, the demand had far exceeded the available supply.
Many new medications see temporary shortages when they first reach the market, but the tirzepatide shortage has lasted much longer and affected far more people than usual. This is partly because the medication became popular faster than expected. Doctors began prescribing it widely, and social media increased awareness of its benefits. At the same time, production of injectable medications like tirzepatide is complex and takes months to scale up. Manufacturing delays, shortages of raw materials, and strict quality controls have all made it hard for the company to keep up.
The shortage has led to real problems for patients. People who rely on tirzepatide to control their blood sugar or maintain weight loss have faced interruptions in their treatment. Missing doses or switching between dosages can cause symptoms like unstable blood sugar, fatigue, or increased hunger. Some people may feel frustrated or anxious about losing progress they have worked hard to achieve. For healthcare providers, the shortage means spending more time managing medication access instead of focusing on care.
Pharmacies have also struggled to meet patient needs. In some cases, only certain dosages — such as the higher strength pens — are unavailable, while in other areas, nearly all strengths are out of stock. Some large pharmacy chains have implemented allocation systems, giving limited supplies only to patients who have been on tirzepatide for a while. Others are updating availability week by week, depending on deliveries from distributors.
The U.S. Food and Drug Administration (FDA) and other health agencies around the world have acknowledged the issue and listed tirzepatide on official shortage databases. Eli Lilly, the manufacturer of Mounjaro and Zepbound, has said it is working to expand production and improve supply, but the company has warned that shortages could continue until manufacturing fully catches up with demand.
For patients and healthcare providers, the most important step is understanding the situation clearly. This article explains why the tirzepatide shortage happened, how long it may last, and what steps patients can take right now to manage their treatment safely. It also reviews the timeline of events, which dosages are hardest to find, and what efforts are being made to fix the shortage.
By the end of this article, readers will have a clear picture of how this global supply problem developed and what to expect next. The goal is not to offer personal medical advice but to help patients and caregivers understand what is happening, why it matters, and what they can do in the meantime. Clear, trustworthy information is essential — especially when medication shortages affect people’s daily health and quality of life.
What Is Tirzepatide and How Does It Work?
Tirzepatide is a modern injectable medicine used to help manage type 2 diabetes and chronic weight conditions. It has quickly become one of the most discussed medications in healthcare because of how well it lowers blood sugar and helps with weight loss. Tirzepatide is sold under brand names such as Mounjaro (for diabetes) and Zepbound (for obesity and weight management). Both are made by the pharmaceutical company Eli Lilly.
While tirzepatide may seem new, its science builds on years of research into hormones that help control blood sugar and appetite. To understand how tirzepatide works, it helps to look at the two hormones it mimics and how they act inside the body.
The Role of GIP and GLP-1 Hormones
When a person eats, the gut releases special hormones that signal the body to release insulin and manage blood sugar. Two of the most important hormones are GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1). These are called incretins.
- GLP-1 helps the pancreas release insulin when blood sugar rises. It also reduces how much sugar the liver releases and slows down how quickly food leaves the stomach. These actions help lower blood sugar and make people feel full longer.
- GIP has a similar role in insulin release but also helps control fat metabolism and may improve how fat is stored and used in the body.
Most older medications for diabetes focused only on the GLP-1 hormone. Tirzepatide is the first medication that acts on both GIP and GLP-1 receptors at the same time. Because of this dual action, doctors often call tirzepatide a “dual incretin” or “twincretin” therapy.
How Tirzepatide Works Inside the Body
Tirzepatide copies the effects of the natural GIP and GLP-1 hormones but lasts longer in the body. It is given as a once-weekly injection, usually under the skin of the abdomen, thigh, or upper arm. After injection, tirzepatide starts working on several parts of the body:
- Pancreas: It helps the pancreas release more insulin when blood sugar levels are high. At the same time, it reduces the release of glucagon, a hormone that increases blood sugar.
- Liver: By lowering glucagon, tirzepatide decreases how much sugar the liver releases into the bloodstream.
- Stomach: It slows how fast the stomach empties after eating, which helps people feel full longer and may reduce food intake.
- Brain: It acts on appetite centers in the brain, decreasing hunger and cravings.
This combined effect helps control blood sugar levels in people with type 2 diabetes and also leads to significant weight loss in many patients.
Approved Uses of Tirzepatide
The U.S. Food and Drug Administration (FDA) first approved tirzepatide in May 2022 under the brand name Mounjaro for adults with type 2 diabetes mellitus. It is meant to be used along with a healthy diet and exercise to improve blood sugar control.
Later, in November 2023, the FDA approved tirzepatide again under the brand name Zepbound for chronic weight management in adults who are obese or overweight and have at least one weight-related condition (such as high blood pressure, high cholesterol, or type 2 diabetes).
These approvals reflect how tirzepatide can help two major health problems: poor blood sugar control and obesity, both of which are common worldwide.
Differences Between Mounjaro and Zepbound
Although both versions contain the same active ingredient, the main difference lies in their approved use and labeling.
- Mounjaro is prescribed to treat type 2 diabetes and focuses on improving blood sugar control.
- Zepbound is prescribed specifically for weight management, even for patients without diabetes, but who meet certain body mass index (BMI) criteria.
The delivery pens and dosing schedules are similar, but healthcare providers choose the version that matches the patient’s condition and insurance coverage.
Why Tirzepatide Is Considered a Breakthrough
Tirzepatide stands out because it helps many people achieve results that were hard to reach with older medications. Clinical trials have shown:
- Large improvements in blood sugar control (A1C levels), often greater than what was seen with GLP-1–only medications like semaglutide.
- Noticeable weight loss, sometimes over 20% of body weight in patients who stayed on therapy for several months.
Because of this, medical experts see tirzepatide as a major step forward in treating metabolic diseases. It addresses not only blood sugar but also weight, insulin sensitivity, and appetite regulation—all key parts of long-term health for people with diabetes or obesity.
How It Differs from Older Treatments
Older diabetes treatments such as metformin, insulin injections, and sulfonylureas work mainly by increasing insulin or reducing sugar production. These can be effective but often lead to weight gain or low blood sugar (hypoglycemia).
Tirzepatide works differently. It helps the body use its natural insulin more efficiently and helps patients lose weight rather than gain it. It also lowers the risk of severe low blood sugar because its insulin-boosting effect happens only when blood sugar levels are high.
Tirzepatide is a once-weekly injectable medicine that mimics two natural gut hormones—GIP and GLP-1—to control blood sugar and reduce appetite. Its dual mechanism makes it one of the most advanced options for people managing type 2 diabetes or obesity. Understanding how tirzepatide works helps explain why so many patients rely on it—and why its shortage is causing significant concern across the medical community.
When Did the Tirzepatide Shortage Begin?
The shortage of tirzepatide did not happen all at once. It developed slowly over time, as demand began to grow faster than supply. Understanding when it began helps explain why the situation has become such a challenge for patients, doctors, and pharmacies around the world.
Early Availability and Rapid Growth
Tirzepatide first gained approval from the U.S. Food and Drug Administration (FDA) in May 2022 under the brand name Mounjaro. It was approved for adults with type 2 diabetes to help improve blood sugar control. Soon after its release, Mounjaro became widely praised for its strong effects on both blood sugar and weight loss. Doctors began prescribing it not only for diabetes management but also for patients with obesity or weight-related health problems, even before official approval for weight loss use.
During its first months on the market, supplies were steady. Pharmacies usually had stock, and patients were able to get their prescriptions filled without delay. However, by late 2022, as news spread about its effectiveness, more and more people began asking for tirzepatide. Social media, television coverage, and word-of-mouth stories about its weight-loss results greatly increased public interest. This sudden jump in demand began to put pressure on manufacturing and distribution channels.
First Signs of Shortage
By early 2023, many pharmacies in the United States started to report that some doses of tirzepatide were becoming harder to find. Patients were told to check back in a few days or weeks. At first, this seemed like a temporary supply issue—something common with new medications that become popular quickly. However, as the months went on, it became clear that the shortage was not limited to a few locations or pharmacies. It was becoming a national issue.
During mid-2023, the FDA officially listed certain strengths of Mounjaro as being in short supply. These included the lower starting doses, such as 2.5 mg and 5 mg, which are most often prescribed for new users who need to begin at a lower level and gradually increase their dose. Because these doses are used to start treatment, the shortage caused a bottleneck—new patients could not begin therapy, and existing patients had difficulty moving up to their next dose.
Zepbound Approval and Demand Spike
In November 2023, the FDA approved tirzepatide under a new brand name, Zepbound, for the treatment of obesity. This approval made tirzepatide officially available for chronic weight management in adults with obesity or overweight with at least one weight-related condition. The approval was widely covered by media and health organizations, leading to an even larger increase in prescriptions.
While the approval of Zepbound was an important step for patients needing weight management therapy, it also put enormous pressure on Eli Lilly, the company that manufactures both Mounjaro and Zepbound. Production facilities were already operating near maximum capacity, and the sudden jump in demand created new delays in supply chains. By the end of 2023, the shortage had spread to most strengths of the medication.
Ongoing Shortage Through 2024
Throughout 2024, the shortage of tirzepatide continued to worsen at different times depending on region and pharmacy system. Some patients reported that they could find one strength but not another, while others had to visit multiple pharmacies or switch temporarily to a different dose. The FDA continued to list tirzepatide as being in limited supply through much of 2024. Many pharmacies began placing limits on how many pens could be dispensed at one time to prevent hoarding and ensure fair distribution among patients.
Eli Lilly released several updates during the year explaining that they were expanding their production capacity by opening new manufacturing lines and partnering with additional suppliers. However, these changes take months or even years to fully implement because producing injectable medications requires strict quality controls and regulatory approvals. This meant that even as manufacturing improvements began, the shortage persisted through the rest of the year.
Global Impact
The shortage was not limited to the United States. Similar reports came from Canada, the United Kingdom, Australia, and parts of Europe, where healthcare providers noted supply gaps and unpredictable delivery times. Some countries delayed or limited access to tirzepatide to ensure that people with type 2 diabetes had priority over those using it for weight loss. The global nature of the shortage showed how interconnected the supply system for modern medicines has become.
Current Situation (as of late 2024 and into 2025)
By the end of 2024 and into 2025, there were signs of gradual improvement, but not a full recovery. Eli Lilly stated that new manufacturing capacity was coming online and that they expected supply to increase during 2025. However, demand for tirzepatide has continued to rise faster than production, especially as more doctors prescribe it for both diabetes and obesity management. Pharmacies still report delays in certain doses, though availability may vary by location and pharmacy chain.
The tirzepatide shortage began in early 2023, grew significantly after the approval of Zepbound in late 2023, and continued throughout 2024 due to overwhelming demand and limited production capacity. The shortage’s timeline shows how quickly medical success stories can lead to unexpected challenges when supply cannot keep pace with need.

What’s Causing the Tirzepatide Shortage?
The shortage of tirzepatide did not happen overnight. It is the result of several overlapping issues that built up over time. Understanding why this medication has become hard to find requires looking at how it is made, how quickly demand grew, and how the supply chain has struggled to keep up.
Below are the main causes explained in clear terms.
Manufacturing Constraints
Tirzepatide is a complex medication to produce. It is a peptide-based drug that requires careful manufacturing in specialized facilities. Unlike common pills, tirzepatide is made as a liquid solution that is delivered through prefilled injection pens.
Each pen must meet strict safety and quality standards before it can be shipped. Producing these pens involves multiple steps — synthesizing the active ingredient, filling the pen, assembling the device, sterilizing, labeling, and packaging.
The main manufacturer, Eli Lilly, operates under high-quality standards set by the U.S. Food and Drug Administration (FDA) and other regulators. Because of this, even a small production issue or delay in one part of the process can affect global supply.
In addition, the company must balance production between two brands that use tirzepatide: Mounjaro (approved for type 2 diabetes) and Zepbound (approved for weight management). Each requires different packaging and labeling, which can slow down production or limit flexibility when supplies run short.
Another challenge is scaling up production lines. Building or expanding a manufacturing plant for injectable medications can take months or even years. Even when new facilities are added, they must pass inspection before they can operate at full capacity. These realities make it difficult to increase output quickly, even when demand surges.
Rapid Surge in Demand
Perhaps the biggest driver of the shortage is the enormous rise in demand. When tirzepatide was first approved for type 2 diabetes in 2022, demand already grew faster than expected. Soon after, studies showed its effectiveness for weight loss, leading to its approval as Zepbound in 2023.
Millions of people, including those without diabetes, became interested in the drug for weight management. Social media and celebrity discussions accelerated awareness, often highlighting dramatic results. As a result, physicians began receiving far more patient requests than supply could handle.
This sudden, global surge in use outpaced the manufacturer’s forecasts. Pharmacies began reporting backorders, and patients who had stable prescriptions found themselves unable to refill doses. Because tirzepatide must be taken regularly, interruptions have a direct impact on treatment outcomes.
Regulatory and Distribution Bottlenecks
Even when production improves, getting tirzepatide from factories to pharmacies involves a complex distribution chain. The drug must be stored and transported at controlled temperatures, known as a “cold chain.” Any delay or shortage in this network — such as limited refrigerated trucks, warehouse capacity, or customs clearance — can slow delivery.
Regulatory approvals also play a role. Every country where tirzepatide is sold requires its own quality review and labeling approval. These steps protect public health but also add time before new batches reach patients. When supplies are tight, a small delay in one region can leave other markets short as well.
Changes in Prescribing Trends
Many healthcare providers are now prescribing tirzepatide to a broader group of patients. This includes individuals with obesity but no diabetes, following recent clinical approvals. Because the medication has shown significant benefits, demand among general practitioners, endocrinologists, and weight-loss clinics has expanded.
At the same time, insurance coverage and direct-to-consumer telehealth services have made access easier in some areas. More patients can now get prescriptions online, which adds pressure to local pharmacies that may not have anticipated the extra demand.
Social Media and Public Awareness
Social media platforms have played a major role in driving public interest. Posts about dramatic weight loss results, often using brand names like Mounjaro or Zepbound, have gone viral. Even though these stories are not always medically balanced, they influence consumer behavior and increase the number of people asking their doctors about tirzepatide.
This rapid growth in public awareness can shift demand faster than manufacturers can respond. As more people seek the medication — sometimes for off-label or cosmetic purposes — the available supply for patients with medical necessity, such as those with type 2 diabetes, becomes strained.
Global Supply Chain Pressures
Finally, the global medicine supply chain is still recovering from the pandemic years. There are still shortages of materials such as glass vials, plastic pen components, and specialized packaging used for injectables. Shipping delays, labor shortages, and increased costs for raw materials have all added pressure to pharmaceutical logistics.
Tirzepatide relies on a coordinated network of suppliers across different countries. If even one supplier faces a delay — for example, in producing a critical component — the entire system slows down. Unlike pills, which can be stockpiled in bulk, injectable pens have more limited storage and shelf life, which makes inventory management harder.
Which Tirzepatide Doses and Products Are Most Affected?
The tirzepatide shortage has not affected every dose or product in the same way. Patients and doctors have noticed that some versions of the medication are much harder to find than others. Understanding which strengths are in short supply—and why—can help patients plan refills, talk with their healthcare providers early, and know what to expect when they visit the pharmacy.
Tirzepatide Comes in Several Doses
Tirzepatide is available in six dose strengths:
- 2.5 mg
- 5 mg
- 7.5 mg
- 10 mg
- 12.5 mg
- 15 mg
Each dose comes as a prefilled injection pen that patients use once a week. The 2.5 mg dose is the starting dose, while the higher doses are used as patients slowly increase over time to reach their “maintenance dose.” This gradual increase helps the body adjust and reduces side effects such as nausea or stomach upset.
Lower Doses Are Especially Hard to Find
During most of the shortage, the 2.5 mg and 5 mg pens have been the most difficult to locate. These are the doses that nearly every new patient begins with, whether the drug is prescribed for diabetes (under the brand name Mounjaro) or for weight management (under Zepbound). Because all patients start with these smaller doses, the demand for them is much higher than for the upper levels.
In addition, patients already taking tirzepatide sometimes need to step back down temporarily if their supply of higher doses runs out or if they experience side effects. This pattern adds even more strain on the lowest strengths, which can cause “cascading shortages” that ripple through all dose levels.
Higher Doses Are Also Becoming Limited
As the shortage continued through 2024 and into 2025, many pharmacies also began reporting low supplies of 7.5 mg, 10 mg, and 12.5 mg pens. While these doses were not as limited in the early months, production and distribution delays eventually affected them too.
One reason is that manufacturing lines often produce all strengths using shared equipment. When lower-dose pens face delays, it slows the entire production cycle. Also, patients who cannot find the correct next step in their dose escalation sometimes remain on the lower dose longer, which increases overall consumption and prolongs shortages across multiple strengths.
The 15 mg dose, which is typically used for maintenance once patients have been on the drug for several months, has been more available at times. However, even the highest strength has had temporary backorders in some regions, particularly through retail pharmacies and mail-order systems.
Mounjaro vs. Zepbound Availability
Although Mounjaro (for type 2 diabetes) and Zepbound (for chronic weight management) both contain tirzepatide, their supplies are managed under slightly different product names and approval pathways.
- Mounjaro was the first to launch in 2022 for diabetes management.
- Zepbound was later approved in 2023 for obesity treatment.
Because both drugs rely on the same active ingredient, they are produced in similar facilities and often face the same shortages. However, distribution channels differ. Pharmacies that serve mainly diabetic patients may receive Mounjaro shipments first, while those filling weight management prescriptions may stock more Zepbound pens.
In some cases, patients have asked about switching between the two products, but this is not recommended without medical approval. Even though they are chemically identical, they are labeled differently, and insurance coverage rules vary. Some insurance plans cover Mounjaro for diabetes but not Zepbound for weight loss, making access even more complicated during shortages.
Geographic Differences in Availability
The severity of the shortage can also depend on where a patient lives.
- Urban areas with higher patient demand often run out faster, as local pharmacies may serve hundreds of tirzepatide prescriptions per week.
- Rural regions may have smaller inventories but sometimes receive shipments that last longer because fewer patients are competing for the same doses.
Mail-order and specialty pharmacies have also reported uneven supplies. Some national chains temporarily limit the number of pens shipped per prescription or prioritize certain patient groups, such as those with diabetes, over those using the medication for weight loss.
Why the Dosing Structure Makes the Shortage Worse
Another important factor is the step-up dosing schedule required for tirzepatide. Every new patient must move through multiple dose levels, increasing roughly every four weeks. When one step in this sequence is unavailable, patients cannot safely progress to the next. For example, if someone cannot find the 5 mg dose after finishing their 2.5 mg pens, they often must pause treatment or restart the ramp-up phase later. This careful titration process means that missing even one dose size can interrupt treatment for weeks or months.
Manufacturing limits also affect this cycle. Because all dose strengths are packaged separately, a shortage of one cannot be replaced with another. Pharmacies cannot divide higher-dose pens or adjust quantities to make up for missing stock.
Current Status and Patient Impact
As of late 2025, reports suggest that lower-dose tirzepatide pens (2.5 mg and 5 mg) remain the most consistently backordered. Mid-range strengths (7.5 mg and 10 mg) are limited but may be available intermittently depending on location. The highest doses (12.5 mg and 15 mg) are somewhat easier to find but still not stable in supply.
For patients, this means:
- Delayed dose escalation.
- Missed or postponed injections.
- Increased frustration during refills.
- The need for closer communication with healthcare providers to manage interruptions safely.
Until production fully catches up with demand, both doctors and patients will need to plan ahead carefully, request refills early, and remain flexible with timing and pharmacy options.
How the Tirzepatide Shortage Is Affecting Patients and Providers
The ongoing shortage of tirzepatide has created serious challenges for both patients and healthcare providers. This medicine, sold under brand names like Mounjaro and Zepbound, has become a key treatment for people with type 2 diabetes and for those who are trying to manage obesity under medical supervision. When access to such a medication becomes limited, it affects daily health routines, blood sugar control, and long-term treatment plans.
This section explains how the shortage impacts patients’ physical health, emotional well-being, and medical care, as well as how it complicates the work of doctors, pharmacists, and clinics that depend on steady supplies to treat their patients safely.
Impact on Patients With Type 2 Diabetes
For people with type 2 diabetes, tirzepatide is often prescribed to help lower blood sugar levels and reduce the risk of heart and kidney problems. It can also help with weight loss, which improves insulin sensitivity. When a patient cannot get their next dose or must skip several weeks of medication, their blood glucose may rise, sometimes quickly. This can lead to higher A1C levels, increased thirst, fatigue, and in severe cases, emergency medical issues such as hyperglycemia or diabetic ketoacidosis.
Because tirzepatide is typically injected once a week, even short interruptions can throw off a patient’s routine. Some people may try to stretch their doses or switch to smaller doses that are more available, but these changes should only be done under a doctor’s supervision. Otherwise, inconsistent use can lead to unpredictable blood sugar control and poor treatment outcomes.
Another concern is emotional stress. Patients who have worked hard to bring their blood sugar levels under control often feel anxious or frustrated when they cannot get their medication. This can create feelings of helplessness and worry about long-term health. Many diabetes educators and clinics are reporting increased calls from patients who are scared or unsure what to do next.
Impact on People Using Tirzepatide for Weight Management
Tirzepatide was approved more recently for chronic weight management under the brand name Zepbound. It helps people reduce appetite and improve metabolic health when combined with diet and exercise. However, during this shortage, many patients have had to pause their treatment or wait weeks for refills.
When therapy is interrupted, appetite and hunger levels may increase again, and weight regain can occur. This is not because the medication “stops working,” but because its effects wear off when it is not taken regularly. The psychological impact of weight regain can be discouraging, particularly for those who have seen strong results and improved confidence.
Healthcare professionals also worry that sudden breaks in treatment could affect motivation and long-term adherence. Many patients may feel that their efforts have been undone, which can lead to stopping medical care altogether or turning to unverified online products that claim to contain tirzepatide — a dangerous and growing problem.
How Providers Are Managing the Shortage
Doctors, nurses, and pharmacists are also under strain from the shortage. Many providers now spend extra time each week tracking pharmacy inventories, rewriting prescriptions, or explaining to patients why their usual medication is unavailable. This adds stress and takes time away from other patients.
Physicians are being forced to prioritize prescriptions — for example, giving existing stock first to patients with type 2 diabetes, since the risks of uncontrolled blood sugar can be life-threatening. Patients using tirzepatide for weight management may be asked to pause or delay starting therapy until supplies improve.
Pharmacists, meanwhile, are facing difficult conversations with customers daily. Some pharmacies keep waitlists, while others rotate supply among clinics. Communication gaps between manufacturers, wholesalers, and pharmacies have made it hard to predict when restocks will happen. This lack of clarity frustrates both patients and medical teams.
Providers must also deal with clinical uncertainty. If a patient has missed several weeks of medication, the doctor may need to restart at a lower dose to prevent side effects like nausea or vomiting. This complicates treatment plans and delays progress.
The Broader Impact on the Healthcare System
The tirzepatide shortage shows how fragile medication supply chains can be. Healthcare systems depend on steady production to manage chronic conditions. When one essential drug becomes limited, demand often shifts toward other medications, creating new shortages elsewhere. This ripple effect strains pharmacies, insurance systems, and medical practices that are already under pressure.
In addition, providers are spending more time verifying that patients are not turning to online or counterfeit sources of tirzepatide. Scammers have begun selling unapproved versions through social media or unlicensed websites. Doctors and pharmacists now play an even more important role in educating patients about safety and encouraging them to use only verified pharmacies.
The tirzepatide shortage has disrupted treatment for thousands of people living with diabetes and obesity. Patients are facing health risks from missed doses, emotional stress from treatment interruptions, and uncertainty about when their medication will return to normal supply. Healthcare providers are doing their best to guide patients safely, but they too are challenged by unpredictable inventories and increased workload.
Until the supply improves, strong communication between patients, providers, and pharmacists will remain essential to maintaining safety and continuity of care.
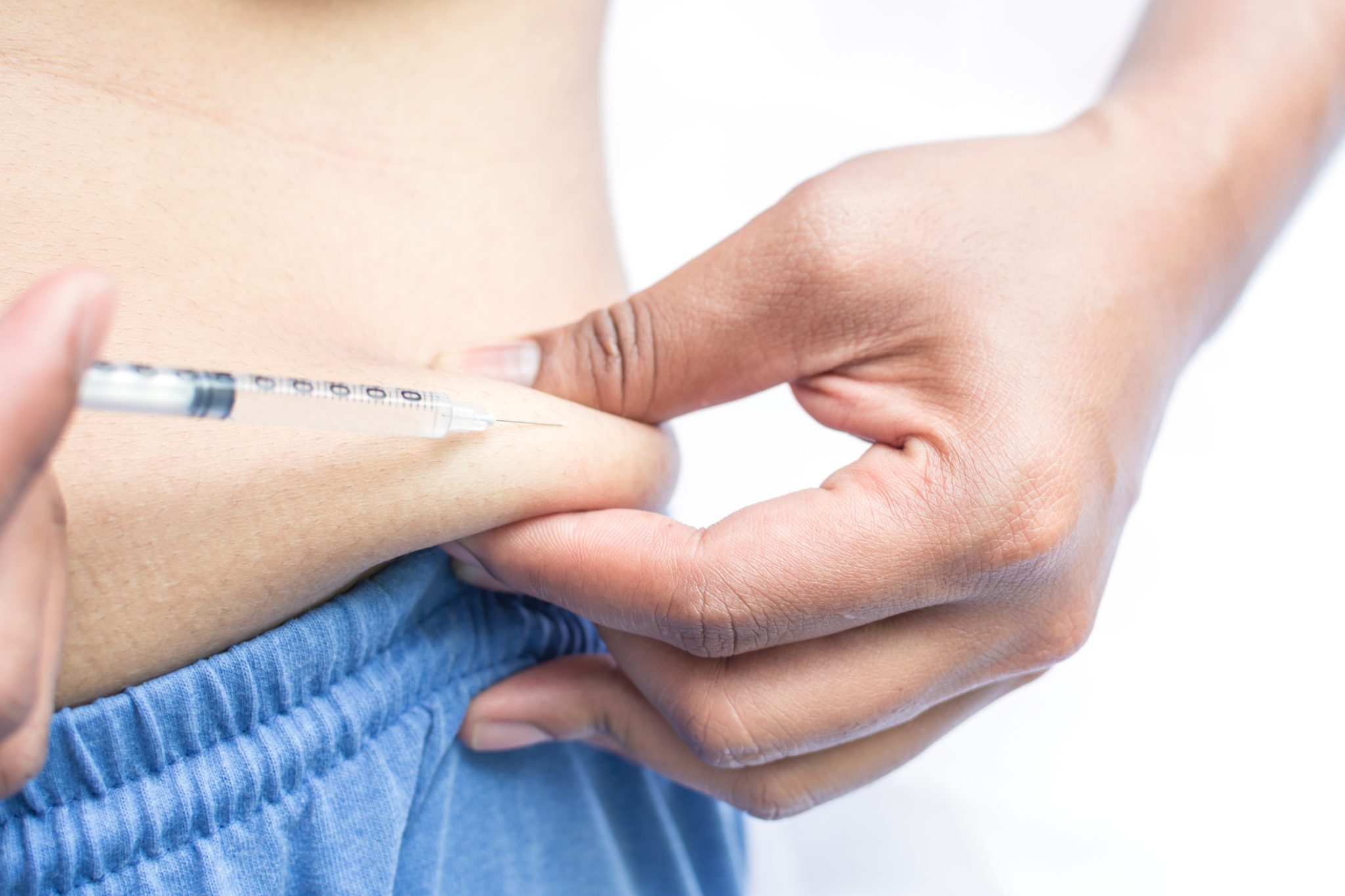
What Is Eli Lilly Doing to Address the Tirzepatide Shortage?
Eli Lilly, the company that makes tirzepatide (sold under the brand names Mounjaro for type 2 diabetes and Zepbound for weight management), has publicly recognized the ongoing shortage and is taking several steps to fix the problem. Because tirzepatide has become one of the most in-demand prescription medicines in recent years, the company has been under heavy pressure from patients, doctors, and regulators to increase supply and improve distribution. Below is a detailed look at what Eli Lilly is doing, how it is expanding production, and what timelines it has shared.
Expanding Manufacturing Capacity
The main reason behind the shortage is that demand for tirzepatide has grown much faster than expected. Eli Lilly has stated that it is building new manufacturing lines and facilities to meet this demand. These include major investments in the United States and in Europe to produce the injectable pens used to deliver tirzepatide safely and accurately.
- New Plants: In 2023 and 2024, Eli Lilly began constructing and expanding multiple sites in Indiana and North Carolina, as well as in Ireland and Germany. Each site must meet strict quality and safety standards set by the U.S. Food and Drug Administration (FDA) and other regulators before producing medicine for patients.
- Device Assembly: Making tirzepatide is complex because it requires both drug manufacturing and pen assembly. The injectable pens are highly precise devices that deliver a measured dose under the skin. Any slowdown in assembly or inspection can delay shipments.
- Automation and Technology: Lilly has said that it is investing billions of dollars in automated equipment and digital tracking systems to speed up production without lowering quality. These upgrades take time, but they are meant to ensure a stable supply over the long term.
Together, these manufacturing projects are expected to increase output gradually through 2025 and beyond.
Prioritizing Certain Dose Strengths
Eli Lilly has also adjusted how it makes and distributes different dose strengths. Some tirzepatide doses—such as the 2.5 mg starting dose—are in especially high demand because every new patient begins treatment at that level. To manage this, Lilly has chosen to prioritize lower doses so that more patients can start therapy, even if higher doses are temporarily limited.
At the same time, Lilly is working with pharmacies and distributors to balance stock across all six dose strengths (2.5 mg to 15 mg). The company’s goal is to reduce interruptions when patients move from one dose to the next. According to official updates, this process is being closely monitored each month, and production plans are adjusted based on where shortages are most severe.
Collaborating With Regulators and Health Agencies
The company is also working with the FDA and other national health authorities to communicate supply information clearly. Lilly provides data on current production, estimated refill timelines, and anticipated recovery dates for each dose strength. This transparency helps doctors and pharmacies plan ahead.
Regulators in the United States, Canada, and the European Union have created public online databases where drug shortages are listed. Eli Lilly regularly updates these databases to show which tirzepatide strengths are affected and when the situation may improve. These reports help prevent misinformation and allow patients to verify the official status instead of relying on social media rumors.
Expanding Distribution and Supply Chain Efficiency
In addition to manufacturing, Eli Lilly has been improving its distribution network. The company partners with large pharmacy wholesalers and logistics companies to move medicine faster once it leaves the factory. Supply-chain adjustments include:
- Better forecasting: Using real-time data from pharmacies to predict which regions are running low.
- Regional allocation: Sending more doses to areas where demand is highest or where critical-care patients are waiting.
- Cold-chain management: Ensuring that shipments stay within the required temperature range, which is essential for injectable medicines.
These efforts aim to shorten the time between production and pharmacy shelves and to avoid sudden stock-outs in specific states or countries.
Preventing Counterfeits and Unsafe Products
Because the shortage has led some people to search for tirzepatide online, Eli Lilly is warning patients about counterfeit or compounded versions sold through unauthorized websites or social-media ads. The company is cooperating with the FDA, customs authorities, and pharmacy boards to track and remove illegal or unsafe products. Lilly reminds patients to buy only from licensed pharmacies and to report suspicious products immediately.
Public Communication and Patient Updates
Eli Lilly has created official shortage-update pages on its website and through press releases. These updates list which tirzepatide doses are available and when restocking is expected. Health-care professionals can also sign up for alerts through their provider networks. The company has encouraged patients to speak with their doctors instead of switching medications or changing doses on their own.
In addition, Eli Lilly has worked with diabetes and obesity-care organizations to share clear, factual information so patients understand what is happening and how long disruptions may last. The focus is on safety and continuity of care.
Expected Timeline for Recovery
While Eli Lilly has not promised an exact end date for the shortage, the company has indicated that supply will improve gradually through 2025 as new production lines open and existing plants reach full capacity. The timeline depends on equipment installation, regulatory approval, and the ongoing rise in global demand.
Most experts expect that supply stability will take several more quarters before pharmacies can keep all doses consistently in stock. Eli Lilly continues to say that its goal is a steady, reliable supply for all patients worldwide.
Eli Lilly’s response to the tirzepatide shortage involves major manufacturing expansion, dose-production prioritization, regulatory cooperation, supply-chain improvements, and strong public communication. The company is investing heavily to make more medicine and deliver it more efficiently while maintaining strict safety and quality standards. Although shortages are still present, these coordinated efforts are expected to ease the problem over time and restore patient access as manufacturing capacity catches up with demand.
What Are Health Authorities and Pharmacies Doing?
As the tirzepatide shortage continues, both health authorities and pharmacies are working to reduce the impact on patients. This effort involves close cooperation between the drug manufacturer (Eli Lilly), the U.S. Food and Drug Administration (FDA), pharmacies, and international agencies such as the European Medicines Agency (EMA). Each group has a specific role in making sure patients stay safe, supplies are used fairly, and counterfeit or unsafe products do not reach the public.
FDA and EMA Monitoring Efforts
In the United States, the FDA Drug Shortage Staff monitors national supply levels of tirzepatide, which includes both Mounjaro (approved for type 2 diabetes) and Zepbound (approved for weight management). The FDA keeps a public list of all current drug shortages and updates it when new information becomes available. When manufacturers like Eli Lilly report production delays or limited stock, the FDA confirms and posts the information on its website so patients and healthcare providers can stay informed.
The FDA’s role is not only to report shortages but also to help prevent unsafe practices during supply gaps. For example, it works with customs and law enforcement to block unapproved or counterfeit versions of tirzepatide that might appear online. Some unregulated products falsely claim to be the same as Mounjaro or Zepbound but may contain unsafe ingredients. The FDA urges patients to buy prescription medications only from licensed pharmacies in the United States.
In Europe, the European Medicines Agency (EMA) and national drug authorities track tirzepatide availability in similar ways. The EMA’s shortage coordination group communicates directly with manufacturers and national health systems to balance distribution across countries when supplies are limited. In some cases, European governments can temporarily authorize alternative packaging or labeling to keep stock moving safely through pharmacies while official packaging catches up.
Pharmacy-Level Actions and Allocation Systems
Pharmacies are on the front line of the shortage. Most major retail chains—such as CVS, Walgreens, and Walmart—receive limited weekly shipments of tirzepatide pens. Because supplies are tight, many have set up allocation systems, which means they must spread available doses across multiple locations and prioritize patients who already have active prescriptions.
This system helps prevent one area or store from using up all available stock. Pharmacists are also encouraged to work closely with prescribing physicians to manage patient expectations. If a patient’s regular dose or strength is unavailable, the pharmacist may contact the healthcare provider to discuss whether a temporary adjustment in dosing schedule is possible or medically appropriate. However, such changes should never be made without professional guidance.
Some pharmacies have started automated refill reminder programs to alert patients when stock arrives. These alerts can help patients refill their prescriptions as soon as possible, especially for doses that are in highest demand. Smaller independent pharmacies may also participate in regional drug-sharing agreements, where neighboring pharmacies share limited supplies to make sure no patient is completely left without medication.
Ensuring Safe and Legal Distribution
Another key focus during the shortage is preventing illegal sales and counterfeit products. Health agencies and pharmacy boards have issued repeated warnings about online advertisements that promise “discount” or “generic” tirzepatide. Many of these products are not genuine and may pose serious health risks. Real tirzepatide pens can only be distributed by licensed pharmacies with a valid prescription.
To help identify legitimate sources, the FDA recommends using the BeSafeRx program, which provides a searchable list of verified online pharmacies. In addition, patients can check whether the pharmacy displays a physical address in the United States and requires a prescription from a licensed clinician—both are signs the business is legitimate.
Pharmacies are also working to educate patients on how to spot fake products. Real Mounjaro and Zepbound pens come in sealed boxes with clear labeling, a printed expiration date, and a manufacturer’s logo. Patients who receive a product that looks different from what they normally use should stop using it and contact their pharmacist or healthcare provider immediately.
Communication and Transparency
Clear communication between pharmacists, doctors, and patients is one of the most important parts of managing the shortage. Pharmacists are trained to explain current supply limits in simple terms and provide updates about when stock might be available again. Health authorities such as the FDA and EMA encourage this open communication to help reduce frustration and prevent unsafe substitutions or self-dosing errors.
Eli Lilly also provides supply updates through healthcare channels and professional organizations. Many pharmacists receive these updates directly from the company or from state boards of pharmacy. This helps ensure that information given to patients is accurate and up to date.
Global Coordination and Next Steps
Globally, the tirzepatide shortage has shown how important coordination is between manufacturers, regulators, and distributors. The FDA and EMA continue to review the manufacturer’s plans for increasing production. They may approve temporary manufacturing expansions or packaging changes to speed up delivery to patients. In some cases, the agencies may allow certain doses to be prioritized for diabetes treatment before weight management use, to protect those who rely on the drug for glucose control.
As supply stabilizes, health authorities will keep monitoring data on how quickly production meets demand. They may also conduct post-shortage reviews to identify lessons that can prevent similar shortages in the future.
Health authorities and pharmacies are responding to the tirzepatide shortage through careful monitoring, fair distribution, and strong communication. The FDA and EMA are tracking supply, blocking counterfeit drugs, and supporting manufacturing solutions. Pharmacies are managing limited stock responsibly, warning patients about unsafe online sellers, and keeping communication open with prescribers and patients.
While these efforts cannot end the shortage overnight, they help ensure that every available dose reaches those who need it most—and that patients stay safe until full supply returns.

What Patients Can Do During the Shortage
The tirzepatide shortage has created stress and uncertainty for many people who rely on this medicine to manage type 2 diabetes or to support weight loss. It can be frustrating to hear that your prescription cannot be filled or that you must wait weeks for your next dose. However, there are practical steps you can take to stay safe, manage your condition, and prepare for changes until the shortage improves.
This section explains what to do if you are already using tirzepatide, starting it for the first time, or looking for trustworthy information about availability.
Contact Your Healthcare Provider Early
If you know your next refill is coming up soon, do not wait until you run out. Contact your healthcare provider or pharmacist at least two to three weeks before your next dose is due. Some pharmacies may have limited stock or may need to order your medication from another location. Early communication gives your provider time to check availability, adjust your dose if necessary, or discuss a temporary plan.
Doctors may sometimes change your dosing schedule or prescribe a different strength that is still available. For example, if your usual 10 mg pen is out of stock, your provider might guide you on how to safely use a lower or higher dose temporarily. Do not make this change on your own. Tirzepatide dosing must be adjusted carefully to avoid side effects such as nausea, low blood sugar, or vomiting.
If your healthcare provider works with a large health system, they may also have access to pharmacy inventory updates and can help you find a pharmacy that still has limited supply.
Manage Missed or Delayed Doses Safely
If you miss a dose because you cannot find the medication, follow the instructions in your medication guide or from your healthcare provider. In general, tirzepatide can be taken up to four days late. After that, you may need to skip the missed dose and continue on your regular schedule.
Avoid taking extra doses to “catch up.” Taking too much can cause side effects such as nausea, vomiting, diarrhea, or dangerously low blood sugar. Keep a record of your doses so you can share it with your doctor. If you have diabetes, check your blood sugar regularly and note any unusual changes. If your levels start to rise, let your healthcare provider know right away.
Monitor Your Health Closely
During a shortage, your access to medication may not be steady. Because of that, it’s important to monitor your body and how you feel. For people with type 2 diabetes, check your blood sugar more often, especially if you’ve had to delay or change your dose. Write down your readings, meals, and any symptoms such as fatigue, excessive thirst, or blurry vision.
For those taking tirzepatide for weight management, keep track of your appetite, weight changes, and digestive symptoms. While you may see some changes during the interruption, your doctor can help guide you back to stability once the medication is available again.
Avoid Buying from Unverified or Online Sources
Because demand is high, there has been an increase in counterfeit and unapproved versions of tirzepatide being sold online. Some websites or social media sellers claim to have Mounjaro or Zepbound available without a prescription. These products may be fake, contaminated, or unsafe. Using unverified medication can cause serious harm.
Always use a licensed pharmacy. To confirm if a pharmacy is legitimate in the U.S., check with your state’s Board of Pharmacy or look for verification through the FDA’s BeSafeRx program. If the price seems too low or if the seller refuses to provide proof of authorization, it’s best to avoid the purchase altogether.
Your healthcare provider or pharmacist can also verify whether a pharmacy is authorized to dispense tirzepatide.
Stay in Regular Contact with Your Care Team
Your doctor, diabetes educator, or pharmacist can help you manage changes in your treatment plan. They can also alert you when new supply becomes available. Many offices now use electronic messaging systems or patient portals—make sure your contact information is up to date so you can receive notifications quickly.
If you have difficulty finding your medication, ask your healthcare provider to document this in your medical record. This can help with insurance claims or appeals if coverage issues arise when switching doses or locations.
Take Care of Lifestyle and Nutrition
While waiting for your medication, focus on healthy eating, regular exercise, and hydration. These habits can help manage blood sugar and support weight control even without medication. A balanced diet with lean proteins, vegetables, and limited processed sugars can reduce spikes in glucose. Try to keep a consistent routine—skipping meals or over-restricting calories can make it harder to maintain stable blood sugar levels.
If you have access to a nutritionist or diabetes educator, they can help adjust your plan temporarily to support your goals until tirzepatide becomes available again.
Be Cautious About Shared Medication
Some people may offer to share their tirzepatide pens or leftover doses. Even if the offer seems kind, this is unsafe and illegal. Each prescription is personalized based on your medical history, dosage, and monitoring plan. Sharing medications increases the risk of contamination, incorrect dosing, or infection from improper handling. Always use your own prescribed medication and equipment.
Keep Updated with Reliable Sources
The situation is changing as manufacturers increase production. To stay informed, check for updates directly from Eli Lilly’s official website, the FDA Drug Shortages list, or your local pharmacy’s announcements. Avoid relying on social media rumors, which often spread incorrect information about stock levels or release dates.
Healthcare organizations and diabetes advocacy groups may also post verified updates about availability and patient resources. Staying informed helps you plan ahead and reduces anxiety about uncertainty.
During the tirzepatide shortage, patients can take control by communicating early with healthcare providers, monitoring their health carefully, and avoiding unsafe or unapproved products. While the shortage is temporary, patient safety must remain the top priority. By staying connected to reliable medical sources and following professional guidance, you can safely manage your condition until regular supply returns.
When Will the Tirzepatide Shortage End?
Many patients are asking the same question: When will the tirzepatide shortage finally be over? Unfortunately, there is no single date that applies to everyone. The situation depends on several factors — how quickly the manufacturer can increase production, how demand continues to grow, and how well pharmacies and health agencies manage distribution. Let’s look closely at what experts and official sources say about the timeline, what causes the delays, and what signs might show that supply is improving.
Manufacturer’s Production Efforts
Eli Lilly, the company that makes tirzepatide (sold under the brand names Mounjaro for diabetes and Zepbound for weight management), has said publicly that it is expanding its manufacturing capacity. The company announced investments of billions of dollars into new production facilities in the United States and Europe. These plants are being built or upgraded to make injectable medicines more efficiently and in larger quantities.
However, building or expanding a pharmaceutical manufacturing site is not quick or simple. It involves regulatory approval, quality testing, and supply chain coordination for specialized parts — including glass cartridges, injector pens, and sterile packaging. Even after a new plant is ready, it can take months to start full-scale production and deliver products to pharmacies.
Because of this, Eli Lilly has explained that supply will improve gradually, not suddenly. Patients may see some doses returning to shelves before others. Lower doses (like the 2.5 mg or 5 mg pens) may be restocked earlier, while higher doses (such as 10 mg or 15 mg) could remain limited for longer.
Factors That Affect the Timeline
Several overlapping issues are influencing how long the shortage lasts:
- Unprecedented Demand Growth:
Tirzepatide’s popularity has grown faster than expected. Millions of people are now prescribed it for diabetes, and more recently, for obesity. Even with increased manufacturing, the demand still exceeds the available supply. - Global Expansion:
As tirzepatide becomes approved in more countries, global demand rises. Eli Lilly has to divide limited supplies across many regions, balancing fairness and regulatory agreements. - Production Complexity:
Tirzepatide is a biologic medication, not a simple chemical pill. That means it’s made through a complex process using living cells. Every batch must meet strict purity and safety standards. A single quality issue can delay thousands of doses. - Component Shortages:
Shortages of injector pen parts and sterile packaging materials also slow down production. These parts are often made by third-party suppliers who must meet pharmaceutical-grade standards. - Regulatory Oversight:
Every time Eli Lilly modifies a manufacturing process, the FDA and other health agencies must review and approve it. This step ensures safety and consistency but can extend the timeline.
What the Forecasts Say
Eli Lilly has indicated that the shortage could improve steadily throughout late 2025, but full resolution may take longer depending on demand trends. In some areas, specific doses may become more consistently available within months, while others could remain difficult to find.
In 2024 and 2025, the company began to open new manufacturing lines and hire additional workers to speed production. Analysts believe that these steps should gradually ease supply pressure. Still, because patient interest in both diabetes and weight management uses continues to grow rapidly, some experts think limited availability could persist into 2026 before stabilizing worldwide.
Pharmacies often report that restocks arrive in small batches and sell out quickly. This pattern suggests that while supply is improving, it is not yet meeting the very high demand.
How to Recognize Signs of Improvement
Patients and healthcare providers can look for these key indicators that the shortage is easing:
- More frequent restocks: Pharmacies start receiving shipments more regularly, and refill requests are filled without long delays.
- Broader dose availability: Multiple tirzepatide strengths, especially higher doses, return to shelves.
- Fewer backorder notices: Distributors stop marking tirzepatide pens as “out of stock” or “temporarily unavailable.”
- Public updates from Eli Lilly: The manufacturer announces that production output has doubled or that new facilities are operational.
- Regulatory reports: The FDA removes tirzepatide from its official drug shortage list, which tracks medications with supply problems.
It is important to note that improvement might not happen evenly everywhere. Some pharmacies or regions could regain steady stock sooner than others depending on distribution logistics and patient density.
What Patients Should Expect
In the coming months, patients may notice gradual improvement, but intermittent shortages are still likely. Doctors might need to adjust refill schedules or dosing plans to match what’s available. Pharmacists may continue to receive limited supplies and prioritize existing patients who are already on tirzepatide treatment.
Eli Lilly continues to advise patients not to switch or alter doses without medical supervision. The company is also encouraging healthcare providers to report availability problems so they can better track regional gaps.
The shortage is unlikely to last forever. With ongoing investments, increased manufacturing, and global coordination, the supply situation should improve step by step. However, high demand for diabetes and weight management treatments means that tirzepatide will remain a closely watched medication for the foreseeable future.
For now, patients should stay in contact with their healthcare providers, follow official updates from the FDA and Eli Lilly, and avoid buying from unverified online sellers claiming to have “extra” stock. Transparency, patience, and clear communication between patients, doctors, and pharmacies are key until supply catches up with demand.
Conclusion
The ongoing shortage of tirzepatide has created frustration, confusion, and worry among many patients and healthcare providers. Tirzepatide, known under brand names like Mounjaro and Zepbound, has changed how doctors treat type 2 diabetes and obesity. Its unique ability to target two important hormones, GIP and GLP-1, has made it very effective for controlling blood sugar and helping with weight loss. Because of this success, demand for the medication has grown much faster than expected. Unfortunately, the demand has been greater than what the current manufacturing and supply systems can handle. The result is a shortage that affects thousands of people who depend on this treatment.
Understanding the causes of the shortage helps explain why it has lasted so long. The main issue is that production has not been able to keep up with global demand. Eli Lilly, the company that makes tirzepatide, has faced challenges expanding its manufacturing capacity quickly enough. Producing injectable drugs like tirzepatide is complex—it requires specialized equipment, strict temperature control, and a stable supply of materials for the injection pens. Even one small disruption in the supply chain, such as a delay in raw materials or packaging, can slow down the entire process. At the same time, demand for tirzepatide has soared due to more people seeking it for weight management in addition to diabetes. This combination—limited production and increasing demand—has placed ongoing pressure on supply.
The shortage has had real effects on patients and healthcare systems. Many people have been unable to fill their prescriptions or have faced long waiting times. Some patients have had to switch doses or skip injections, which can make it harder to control blood sugar or maintain steady progress in weight loss. Doctors and pharmacists are also feeling the pressure. They are trying to prioritize who most urgently needs the medication while explaining the situation to patients who may not fully understand why it is unavailable. For people who have worked hard to manage their health with tirzepatide, these interruptions can be stressful and discouraging.
Eli Lilly has said that it is taking steps to fix the problem. The company has been building more production facilities and increasing staffing to expand output. It has also been working with regulators, including the U.S. Food and Drug Administration (FDA), to speed up approval for new manufacturing lines. These efforts take time because quality and safety standards must be maintained throughout the process. The company has shared timelines suggesting that supply should begin to improve gradually, but there is still uncertainty about exactly when all doses will become widely available again. As of now, some strengths remain harder to find than others, especially the lower starting doses that new patients often need.
Health agencies and pharmacies are also playing a role in managing the shortage. The FDA continues to monitor supply levels and issue updates to the public. Pharmacies have started to use allocation systems to make sure available stock is distributed fairly and reaches those with ongoing prescriptions. Some have begun alert programs that notify patients when their medication is restocked. These steps help reduce hoarding and panic-buying, which can make shortages worse. It is also important for patients to be cautious about purchasing medication from unverified online sellers. Counterfeit versions of tirzepatide have already appeared on the market, posing serious safety risks. Patients should always obtain their prescriptions through trusted healthcare channels.
For individuals currently affected, staying in close contact with their healthcare providers is the most important step. Doctors can help adjust treatment schedules or offer safe temporary dosing plans. They can also monitor for any changes in blood sugar or side effects if injections are delayed. Patients should plan ahead for refills and notify their providers early if they are running low. For new patients who have just started tirzepatide, it may take longer to get consistent access to the medication. It is vital to avoid using leftover doses from other people or to share pens, as this can lead to infection and inaccurate dosing.
While the shortage is expected to improve over time, the exact end date remains uncertain. The timeline depends on how quickly new production capacity becomes operational and whether demand continues to rise. Some analysts predict that supply will stabilize over the coming year as manufacturing scales up and global distribution becomes smoother. However, even when production meets demand, small shortages may continue for specific doses or regions. It will take steady communication between manufacturers, pharmacies, regulators, and patients to ensure consistent access.
In the meantime, knowledge is one of the best tools for managing this situation. Staying informed through official updates from the FDA, Eli Lilly, and trusted healthcare organizations can help patients make good decisions. Clear communication with healthcare providers helps ensure safety and continuity of care. Patients should remember that they are not alone in facing these challenges—thousands of others are experiencing similar difficulties, and the medical community is working hard to restore stable supply.
In summary, the tirzepatide shortage is the result of strong demand, limited manufacturing capacity, and ongoing supply chain issues. It has affected both patients and healthcare providers, but progress is being made to resolve it. Eli Lilly’s expansion efforts and the oversight of health authorities offer hope that the situation will gradually improve. Until then, patients are encouraged to stay alert, plan ahead, and rely on their healthcare teams for guidance. The most important thing is to continue managing their health safely and patiently while waiting for full availability to return.
Research Citations
U.S. Food and Drug Administration. (2025, April 28). FDA clarifies policies for compounders as national GLP-1 supply begins to stabilize.
U.S. Food and Drug Administration. (2024, December 19). Declaratory order: Resolution of shortages of tirzepatide injection products (Mounjaro and Zepbound) [PDF].
U.S. Food and Drug Administration. (2024, December 19). Resolution of tirzepatide injection product shortage and supply status [PDF].
American Society of Health-System Pharmacists. (2023, April 17). Drug Shortage Detail: Tirzepatide injection.
Whitley, H. P., Trujillo, J. M., & Neumiller, J. J. (2023). Special report: Potential strategies for addressing GLP-1 and dual GLP-1/GIP receptor agonist shortages. Clinical Diabetes, 41(3), 467–473. https://doi.org/10.2337/cd23-0023
Altabas, V., Orlović, Z., & Baretić, M. (2025). Addressing the shortage of GLP-1 RA and dual GIP/GLP-1 RA-based therapies—A systematic review. Diabetology, 6(6), 52. https://doi.org/10.3390/diabetology6060052
Sood, N., Whaley, C. M., & Emond, S. K. (2025). Global rise of compounded weight-loss medicines. Journal of the Endocrine Society, 9(8), bvaf084. https://doi.org/10.1210/jendso/bvaf084
Nanayakkara, N., Huang, M. L., Jenkins, A. J., & Cohen, N. D. (2024). The impact of GLP-1 receptor agonist shortages on glycaemic control: Findings from an Australian specialist diabetes clinic. Diabetes Research and Clinical Practice, 213, 111740. https://doi.org/10.1016/j.diabres.2024.111740
Pearson, S. D., Whaley, C. M., & Emond, S. K. (2025). Affordable access to GLP-1 obesity medications: Strategies to guide market action and policy solutions in the U.S. Journal of Comparative Effectiveness Research, 14(9), e250083. https://doi.org/10.57264/cer-2025-0083
DiStefano, M. J., et al. (2024). Compounded glucagon-like peptide-1 receptor agonists for weight loss: The direct-to-consumer market in Colorado. Journal of Pharmaceutical Policy and Practice, 17, Article 115. https://doi.org/10.1186/s40545-024-00756-2
Questions and Answers: Tirzepatide Shortage
The FDA announced the tirzepatide shortage was resolved on December 19, 2024. Availability has largely returned to normal, though some pharmacies may still experience temporary stock issues.
The shortage mainly affected prefilled pens of Mounjaro (for type 2 diabetes) and Zepbound (for obesity). Some vial formulations were not listed as in shortage.
Demand for tirzepatide increased much faster than production capacity. The manufacturer expanded facilities to meet growing demand.
The FDA determined supply was sufficient to meet demand and ended temporary rules that allowed compounding and other exceptions.
No. Once the shortage ended, the FDA withdrew compounding allowances for tirzepatide under sections 503A and 503B. The agency has also issued warnings about unsafe compounded products.
Contact your prescriber. They may adjust your strength, suggest another pharmacy, or provide a short-term plan. Do not change doses on your own.
It can be, but only under a healthcare provider’s supervision. Medications in this class differ in dosing and side effects.
Use only licensed pharmacies, check packaging carefully, and be cautious of online or “research” versions. The FDA has warned about fraudulent tirzepatide products.
Yes. Eli Lilly expanded its global manufacturing and distribution network to stabilize tirzepatide availability worldwide.
Not entirely. Even though the national shortage has ended, temporary or local supply interruptions may still occur from time to time.

Dr. Jay Flottman
Dr. Jay Flottmann is a physician in Panama City, FL. He received his medical degree from University of Texas Medical Branch and has been in practice 21 years. He is experienced in military medicine, an FAA medical examiner, human performance expert, and fighter pilot.
Professionally, I am a medical doctor (M.D. from the University of Texas Medical Branch at Galveston), a fighter pilot (United States Air Force trained – F-15C/F-22/AT-38C), and entrepreneur.
